Structural determinants of the interaction between the Haemophilus influenzae Hap autotransporter and fibronectin
- PMID: 24687948
- PMCID: PMC4039244
- DOI: 10.1099/mic.0.077784-0
Structural determinants of the interaction between the Haemophilus influenzae Hap autotransporter and fibronectin
Abstract
Haemophilus influenzae is a Gram-negative cocco-bacillus that initiates infection by colonizing the upper respiratory tract. Hap is an H. influenzae serine protease autotransporter protein that mediates adherence, invasion and microcolony formation in assays with human epithelial cells and is presumed to facilitate the process of colonization. Additionally, Hap mediates adherence to fibronectin, laminin and collagen IV, extracellular matrix (ECM) proteins that are present in the respiratory tract and are probably important targets for H. influenzae colonization. The region of Hap responsible for adherence to ECM proteins has been localized to the C-terminal 511 aa of the Hap passenger domain (HapS). In this study, we characterized the structural determinants of the interaction between HapS and fibronectin. Using defined fibronectin fragments, we established that Hap interacts with the fibronectin repeat fragment called FNIII(1-2). Using site-directed mutagenesis, we found a series of motifs in the C-terminal region of HapS that contribute to the interaction with fibronectin. Most of these motifs are located on the F1 and F3 faces of the HapS structure, suggesting that the F1 and F3 faces may be responsible for the HapS-fibronectin interaction.
© 2014 The Authors.
Figures

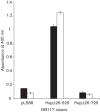
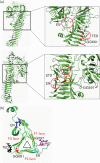

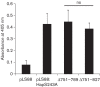
References
-
- Bingham R. J., Rudiño-Piñera E., Meenan N. A., Schwarz-Linek U., Turkenburg J. P., Höök M., Garman E. F., Potts J. R. (2008). Crystal structures of fibronectin-binding sites from Staphylococcus aureus FnBPA in complex with fibronectin domains. Proc Natl Acad Sci U S A 105, 12254–12258. 10.1073/pnas.0803556105 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

