Differential ability of surface and endosomal TLRs to induce CD8 T cell responses in vivo
- PMID: 24688022
- PMCID: PMC4002505
- DOI: 10.4049/jimmunol.1302244
Differential ability of surface and endosomal TLRs to induce CD8 T cell responses in vivo
Abstract
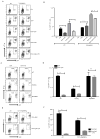
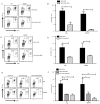
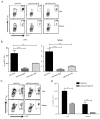
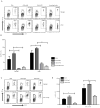
TLR activation on dendritic cells (DCs) induces DC maturation and secretion of proinflammatory cytokines, both of which are important for activation and differentiation of CD4 T cells. The importance of TLR activation on DCs for CD8 T cell responses is less clear. In this study, we tested the ability of different TLRs to regulate CD8 T cell responses to pathogens. We found that although all TLRs are able to induce CD8 T cell activation in vitro, there are profound differences in their ability to activate CD8 T cells in vivo. The nucleic acid recognizing endosomal TLRs, TLR3 and TLR9, had a potent ability to induce CD8 T cell activation. However, the surface TLRs, TLR2 and TLR4, that recognize bacterial ligands were not only incapable of inducing CD8 T cell priming, but they had a dominant effect of inhibiting CD8 T cell expansion induced by activation of endosomal TLRs. We found that TLR2 and TLR4, acting in a MyD88-dependent manner, influenced CD8 T cell priming by altering the composition of DCs in the draining lymph nodes. Our results have important implications for combined bacterial and viral infections and suggest that bacterial infections could constrain the ability of the host to mount effective antiviral CD8 T cell immunity.
Figures

Similar articles
-
Variable requirement of dendritic cells for recruitment of NK and T cells to different TLR agonists.J Immunol. 2007 Mar 15;178(6):3886-92. doi: 10.4049/jimmunol.178.6.3886. J Immunol. 2007. PMID: 17339488
-
Tubulation of endosomal structures in human dendritic cells by Toll-like receptor ligation and lymphocyte contact accompanies antigen cross-presentation.J Biol Chem. 2014 Jan 3;289(1):520-8. doi: 10.1074/jbc.M113.511147. Epub 2013 Nov 14. J Biol Chem. 2014. PMID: 24235148 Free PMC article. Clinical Trial.
-
Synergistic activation of dendritic cells by combined Toll-like receptor ligation induces superior CTL responses in vivo.Blood. 2006 Jul 15;108(2):544-50. doi: 10.1182/blood-2005-10-4015. Epub 2006 Mar 14. Blood. 2006. PMID: 16537810
-
Regulation of dendritic cell function through Toll-like receptors.Curr Mol Med. 2003 Jun;3(4):373-85. doi: 10.2174/1566524033479726. Curr Mol Med. 2003. PMID: 12776992 Review.
-
Cell Surface Expression of Endosomal Toll-Like Receptors-A Necessity or a Superfluous Duplication?Front Immunol. 2021 Feb 1;11:620972. doi: 10.3389/fimmu.2020.620972. eCollection 2020. Front Immunol. 2021. PMID: 33597952 Free PMC article. Review.
Cited by
-
Liver-resident CD103+ dendritic cells prime antiviral CD8+ T cells in situ.J Immunol. 2015 Apr 1;194(7):3213-22. doi: 10.4049/jimmunol.1402622. Epub 2015 Feb 23. J Immunol. 2015. PMID: 25712214 Free PMC article.
-
Innate Control of Adaptive Immunity: Beyond the Three-Signal Paradigm.J Immunol. 2017 May 15;198(10):3791-3800. doi: 10.4049/jimmunol.1602000. J Immunol. 2017. PMID: 28483987 Free PMC article. Review.
-
Modifying the tumor microenvironment using nanoparticle therapeutics.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016 Nov;8(6):891-908. doi: 10.1002/wnan.1406. Epub 2016 Apr 1. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016. PMID: 27038329 Free PMC article. Review.
-
Diminished HIV Infection of Target CD4+ T Cells in a Toll-Like Receptor 4 Stimulated in vitro Model.Front Immunol. 2019 Jul 23;10:1705. doi: 10.3389/fimmu.2019.01705. eCollection 2019. Front Immunol. 2019. PMID: 31396221 Free PMC article.
-
Control of adaptive immunity by pattern recognition receptors.Immunity. 2024 Apr 9;57(4):632-648. doi: 10.1016/j.immuni.2024.03.014. Immunity. 2024. PMID: 38599163 Free PMC article. Review.
References
-
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. - PubMed
-
- Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–1018. - PubMed
-
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nature immunology. 2004;5:987–995. - PubMed
-
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nature immunology. 2004;5:730–737. - PubMed
-
- Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends in immunology. 2005;26:447–454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

