The role of lipolysis in human orosensory fat perception
- PMID: 24688103
- PMCID: PMC3995465
- DOI: 10.1194/jlr.M046029
The role of lipolysis in human orosensory fat perception
Abstract
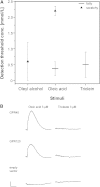
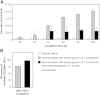
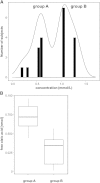
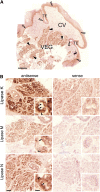
Taste perception elicited by food constituents and facilitated by sensory cells in the oral cavity is important for the survival of organisms. In addition to the five basic taste modalities, sweet, umami, bitter, sour, and salty, orosensory perception of stimuli such as fat constituents is intensely investigated. Experiments in rodents and humans suggest that free fatty acids represent a major stimulus for the perception of fat-containing food. However, the lipid fraction of foods mainly consists of triglycerides in which fatty acids are esterified with glycerol. Whereas effective lipolysis by secreted lipases (LIPs) liberating fatty acids from triglycerides in the rodent oral cavity is well established, a similar mechanism in humans is disputed. By psychophysical analyses of humans, we demonstrate responses upon stimulation with triglycerides which are attenuated by concomitant LIP inhibitor administration. Moreover, lipolytic activities detected in minor salivary gland secretions directly supplying gustatory papillae were correlated to individual sensitivities for triglycerides, suggesting that differential LIP levels may contribute to variant fat perception. Intriguingly, we found that the LIPF gene coding for lingual/gastric LIP is not expressed in human lingual tissue. Instead, we identified the expression of other LIPs, which may compensate for the absence of LIPF.
Keywords: G protein-coupled receptor; free fatty acid; lipase; taste; triglyceride; von Ebner’s gland.
Figures



References
-
- Chandrashekar J., Hoon M. A., Ryba N. J., Zuker C. S. 2006. The receptors and cells for mammalian taste. Nature. 444: 288–294. - PubMed
-
- Gilbertson T. A., Fontenot D. T., Liu L., Zhang H., Monroe W. T. 1997. Fatty acid modulation of K+ channels in taste receptor cells: gustatory cues for dietary fat. Am. J. Physiol. 272: C1203–C1210. - PubMed
-
- Schiffman S. S. 2000. Taste quality and neural coding: implications from psychophysics and neurophysiology. Physiol. Behav. 69: 147–159. - PubMed
-
- Watson K. J., Kim I., Baquero A. F., Burks C. A., Liu L., Gilbertson T. A. 2007. Expression of aquaporin water channels in rat taste buds. Chem. Senses. 32: 411–421. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

