Dissecting complex polyketide biosynthesis
- PMID: 24688670
- PMCID: PMC3962154
- DOI: 10.5936/csbj.201210010
Dissecting complex polyketide biosynthesis
Erratum in
-
Corrigendum to "Dissecting complex polyketide biosynthesis" [Comput Struct Biotechnol J 3(4) (2012) 1-8].Comput Struct Biotechnol J. 2015 Nov 27;14:86. doi: 10.1016/j.csbj.2015.11.003. eCollection 2016. Comput Struct Biotechnol J. 2015. PMID: 27069558 Free PMC article.
Abstract
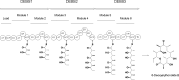
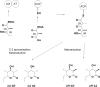
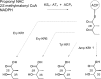
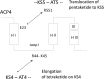
Numerous bioactive natural products are synthesised by modular polyketide synthases. These compounds can be made in high yield by native multienzyme assembly lines. However, formation of analogues by genetically engineered systems is often considerably less efficient. Biochemical studies on intact polyketide synthase proteins have amassed a body of knowledge that is substantial but still incomplete. Recently, the constituent enzymes have been structurally characterised as discrete domains or didomains. These recombinant proteins have been used to reconstitute single extension cycles in vitro. This has given further insights into how the final stereochemistry of chiral centres in polyketides is determined. In addition, this approach has revealed how domains co-operate to ensure efficient transfer of growing intermediates along the assembly line. This work is leading towards more effective re-programming of these enzymes for use in synthesis of new medicinal compounds.
Figures
References
-
- Weissman K (2009) Introduction to polyketide biosynthesis. Meth. Enzymol 459: 3–16 - PubMed
-
- Rix U, Fischer C, Remsing L, Rohr J (2002) Modification of post-PKS tailoring steps through combinatorial biosynthesis. Nat. Prod. Rep. 19: 542–580 - PubMed
-
- Hutchinson E, Murphy B, Dunne T, Breen C, Rawlings B, Caffrey P (2010) Redesign of polyene macrolide glycosylation: engineered biosynthesis of 19-O-perosaminyl-amphoteronolide. B. Chem. Biol. 17: 174–182 - PubMed
-
- Stephens N, Rawlings B, Caffrey P (2012) Streptomyces nodosus host strains optimised for polyene glycosylation engineering. Biosci. Biotechnol. Biochem. 76: 384–387 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
