The essential biology of the endoplasmic reticulum stress response for structural and computational biologists
- PMID: 24688718
- PMCID: PMC3962220
- DOI: 10.5936/csbj.201303010
The essential biology of the endoplasmic reticulum stress response for structural and computational biologists
Abstract
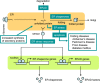
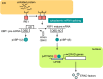
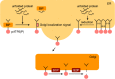
The endoplasmic reticulum (ER) stress response is a cytoprotective mechanism that maintains homeostasis of the ER by upregulating the capacity of the ER in accordance with cellular demands. If the ER stress response cannot function correctly, because of reasons such as aging, genetic mutation or environmental stress, unfolded proteins accumulate in the ER and cause ER stress-induced apoptosis, resulting in the onset of folding diseases, including Alzheimer's disease and diabetes mellitus. Although the mechanism of the ER stress response has been analyzed extensively by biochemists, cell biologists and molecular biologists, many aspects remain to be elucidated. For example, it is unclear how sensor molecules detect ER stress, or how cells choose the two opposite cell fates (survival or apoptosis) during the ER stress response. To resolve these critical issues, structural and computational approaches will be indispensable, although the mechanism of the ER stress response is complicated and difficult to understand holistically at a glance. Here, we provide a concise introduction to the mammalian ER stress response for structural and computational biologists.
Keywords: ATF6; ER stress; ERAD; IRE1; PERK; XBP1; unfolded protein response.
Figures





References
-
- Gething M-J (1997) Guidebook to Molecular Chaperones and Protein-Folding Catalysts. Oxford: Oxford University Press
-
- Imaizumi K, Miyoshi K, Katayama T, Yoneda T, Taniguchi M, et al. (2001) The unfolded protein response and Alzheimer's disease. Biochim Biophys Acta 1536: 85–96 - PubMed
-
- Wang HQ, Takahashi R (2007) Expanding insights on the involvement of endoplasmic reticulum stress in Parkinson's disease. Antioxid Redox Signal 9: 553–561 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
