Ketamine as the prototype glutamatergic antidepressant: pharmacodynamic actions, and a systematic review and meta-analysis of efficacy
- PMID: 24688759
- PMCID: PMC3952483
- DOI: 10.1177/2045125313507739
Ketamine as the prototype glutamatergic antidepressant: pharmacodynamic actions, and a systematic review and meta-analysis of efficacy
Abstract
The burden of depressive disorders and the frequent inadequacy of their current pharmacological treatments are well established. The anaesthetic and hallucinogenic drug ketamine has provoked much interest over the past decade or so as an extremely rapidly acting antidepressant that does not modify 'classical' monoaminergic receptors. Current evidence has shown several ways through which it might exert therapeutic antidepressant actions: blockade of glutamatergic NMDA receptors and relative upregulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) subtypes may alter cortical connectivity patterns; through intracellular changes in protein expression, including the proteins mammalian target of rapamycin (mTOR) and brain-derived neurotrophic factor (BDNF); and alteration of intracellular signalling cascades. The clinical evidence demonstrates rapid improvements in mood and suicidal thinking in most participants, although study numbers have generally been small and many trials are unblinded and methodologically weak. There is a small body of work to suggest ketamine might also augment electroconvulsive therapy and potentially have a role as a surgical anaesthetic in depressed patients. A major problem is that the effects of ketamine appear temporary, disappearing after days to weeks (although longer benefits have been sustained in some), and attempts to circumvent this through pharmacological augmentation have been disappointing thus far. These exciting data are providing new insights into neurobiological models of depression, and potentially opening up a new class of antidepressants, but there are significant practical and ethical issues about any future mainstream clinical role it might have.
Keywords: antidepressant; glutamatergic; ketamine.
Conflict of interest statement
Figures
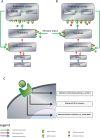

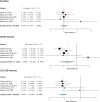
References
-
- aan het Rot M., Collins K., Murrough J., Perez A., Reich D., Charney D., et al. (2010) Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry 67: 139–145 - PubMed
-
- Abe H., Rusak B., Robertson H. (1992) NMDA and non-NMDA receptor antagonists inhibit photic induction of Fos protein in the hamster suprachiasmatic nucleus. Brain Res Bull 28: 831–835 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

