Cardiomyocyte proliferation vs progenitor cells in myocardial regeneration: The debate continues
- PMID: 24689031
- PMCID: PMC3963760
- DOI: 10.5339/gcsp.2013.37
Cardiomyocyte proliferation vs progenitor cells in myocardial regeneration: The debate continues
Abstract
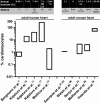
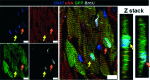
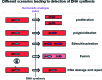
In recent years, several landmark studies have provided compelling evidence that cardiomyogenesis occurs in the adult mammalian heart. However, the rate of new cardiomyocyte formation is inadequate for complete restoration of the normal mass of myocardial tissue, should a significant myocardial injury occur, such as myocardial infarction. The cellular origin of postnatal cardiomyogenesis in mammals remains a controversial issue and two mechanisms seem to be participating, proliferation of pre-existing cardiomyocytes and myogenic differentiation of progenitor cells. We will discuss the relative importance of these two processes in different settings, such as normal ageing and post-myocardial injury, as well as the strengths and limitations of the existing experimental methodologies used in the relevant studies. Further clarification of the mechanisms underlying cardiomyogenesis in mammals will open the way for their therapeutic exploitation in the clinical field, with the scope of myocardial regeneration.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
