Hydrogel scaffolds for tissue engineering: Progress and challenges
- PMID: 24689032
- PMCID: PMC3963751
- DOI: 10.5339/gcsp.2013.38
Hydrogel scaffolds for tissue engineering: Progress and challenges
Abstract
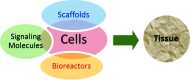
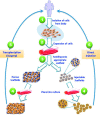
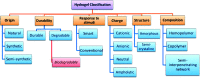
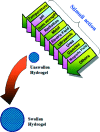
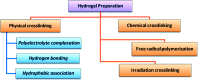
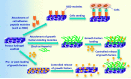
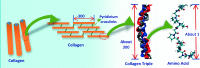
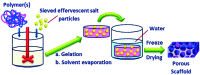
Designing of biologically active scaffolds with optimal characteristics is one of the key factors for successful tissue engineering. Recently, hydrogels have received a considerable interest as leading candidates for engineered tissue scaffolds due to their unique compositional and structural similarities to the natural extracellular matrix, in addition to their desirable framework for cellular proliferation and survival. More recently, the ability to control the shape, porosity, surface morphology, and size of hydrogel scaffolds has created new opportunities to overcome various challenges in tissue engineering such as vascularization, tissue architecture and simultaneous seeding of multiple cells. This review provides an overview of the different types of hydrogels, the approaches that can be used to fabricate hydrogel matrices with specific features and the recent applications of hydrogels in tissue engineering. Special attention was given to the various design considerations for an efficient hydrogel scaffold in tissue engineering. Also, the challenges associated with the use of hydrogel scaffolds were described.
Keywords: bioadhesion; biocompatibility, tissue engineering; biodegradability; hydrogels; scaffolds.
Figures



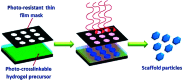
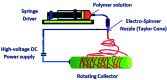
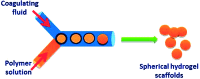

References
-
- Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–492. - PubMed
-
- Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869–1879. - PubMed
-
- Kyung JH, Yeon KS, Jeong KS, Moo LY. pH / temperature-responsive semi-IPN hydrogels composed of alginate and poly(N-isopropylacrylamide) J Appl Polym Sci. 2002;83:128–136.
-
- Wichterle O, Lim D. Hydrophilic gels for biological use. Nature. 1960;185:117–129.
-
- Hoffman AS. Hydrogels for biomedical applications. Ann N Y Acad Sci. 2001;944:62–73. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
