Flow cytometry assessment of in vitro generated CD138+ human plasma cells
- PMID: 24689045
- PMCID: PMC3933014
- DOI: 10.1155/2014/536482
Flow cytometry assessment of in vitro generated CD138+ human plasma cells
Abstract
The in vitro CD40-CD154 interaction promotes human B lymphocytes differentiation into plasma cells. Currently, CD138 is the hallmark marker enabling the detection of human plasma cells, both in vitro and in vivo; its presence can be monitored by flow cytometry using a specific antibody. We have developed a culture system allowing for the differentiation of memory B lymphocytes. In order to detect the newly formed plasma cells, we have compared their staining using five anti-CD138 monoclonal antibodies (mAbs). As a reference, we also tested human cell lines, peripheral blood mononuclear cells, and bone marrow samples. The five anti-CD138 mAbs stained RPMI-8226 cells (>98%) with variable stain index (SI). The highest SI was obtained with B-A38 mAb while the lowest SI was obtained with DL-101 and 1D4 mAbs. However, the anti-CD138 mAbs were not showing equivalent CD138(+) cells frequencies within the generated plasma cells. B-A38, B-B4, and MI-15 were similar (15-25%) while DL-101 mAb stained a higher proportion of CD138-positive cells (38-42%). DL-101 and B-A38 mAbs stained similar populations in bone marrow samples but differed in their capacity to bind to CD138(high) and CD138(lo) cell lines. In conclusion, such cellular fluctuations suggest heterogeneity in human plasma cell populations and/or in CD138 molecules.
Figures

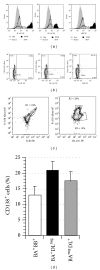
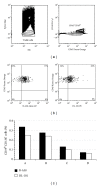
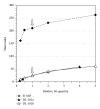
References
-
- Gattei V, Godeas C, Degan M, Rossi FM, Aldinucci D, Pinto A. Characterization of anti-CD138 monoclonal antibodies as tools for investigating the molecular polymorphism of syndecan-1 in human lymphoma cells. British Journal of Haematology. 1999;104(1):152–162. - PubMed
-
- O’Connell FP, Pinkus JL, Pinkus GS. CD138 (Syndecan-1), a plasma cell marker—immunohistochemical profile in hematopoietic and nonhematopoietic neoplasms. The American Journal of Clinical Pathology. 2004;121(2):254–263. - PubMed
-
- Wijdenes J, Vooijs WC, Clément C, et al. A plasmocyte selective monoclonal antibody (B-B4) recognizes syndecan-1. British Journal of Haematology. 1996;94(2):318–323. - PubMed
-
- Costes V, Magen V, Legouffe E, et al. The Mi15 monoclonal antibody (anti-syndecan-1) is a reliable marker for quantifying plasma cells in paraffin-embedded bone marrow biopsy specimens. Human Pathology. 1999;30(12):1405–1411. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

