Advance of molecular imaging technology and targeted imaging agent in imaging and therapy
- PMID: 24689058
- PMCID: PMC3943245
- DOI: 10.1155/2014/819324
Advance of molecular imaging technology and targeted imaging agent in imaging and therapy
Abstract
Molecular imaging is an emerging field that integrates advanced imaging technology with cellular and molecular biology. It can realize noninvasive and real time visualization, measurement of physiological or pathological process in the living organism at the cellular and molecular level, providing an effective method of information acquiring for diagnosis, therapy, and drug development and evaluating treatment of efficacy. Molecular imaging requires high resolution and high sensitive instruments and specific imaging agents that link the imaging signal with molecular event. Recently, the application of new emerging chemical technology and nanotechnology has stimulated the development of imaging agents. Nanoparticles modified with small molecule, peptide, antibody, and aptamer have been extensively applied for preclinical studies. Therapeutic drug or gene is incorporated into nanoparticles to construct multifunctional imaging agents which allow for theranostic applications. In this review, we will discuss the characteristics of molecular imaging, the novel imaging agent including targeted imaging agent and multifunctional imaging agent, as well as cite some examples of their application in molecular imaging and therapy.
Figures
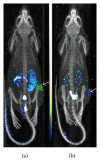


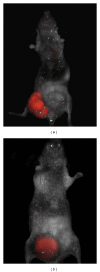

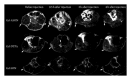
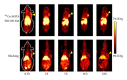
References
-
- Pan D, Lanza GM, Wickline SA, Caruthers SD. Nanomedicine: perspective and promises with ligand-directed molecular imaging. European Journal of Radiology. 2009;70(2):274–285. - PubMed
-
- Atreya R, Goetz M. Molecular imaging in gastroenterology. Nature Reviews Gastroenterology & Hepatology. 2013;10(12):704–7712. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

