Transthyretin-derived peptides as β-amyloid inhibitors
- PMID: 24689444
- PMCID: PMC4102968
- DOI: 10.1021/cn500014u
Transthyretin-derived peptides as β-amyloid inhibitors
Abstract
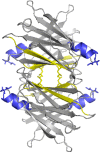
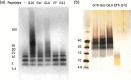
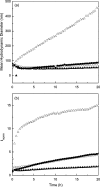
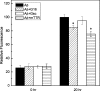
Self-association of β-amyloid (Aβ) into soluble oligomers and fibrillar aggregates is associated with Alzheimer's disease pathology, motivating the search for compounds that selectively bind to and inhibit Aβ oligomerization and/or neurotoxicity. Numerous small-molecule inhibitors of Aβ aggregation or toxicity have been reported in the literature. However, because of their greater size and complexity, peptides and peptidomimetics may afford improved specificity and affinity as Aβ aggregation modulators compared to small molecules. Two divergent strategies have been employed in the search for peptides that bind Aβ: (i) using recognition domains corresponding to sequences in Aβ itself (such as KLVFF) and (ii) screening random peptide-based libraries. In this study, we propose a third strategy, specifically, designing peptides that mimic binding domains of Aβ-binding proteins. Transthyretin, a plasma transport protein that is also relatively abundant in cerebrospinal fluid, has been shown to bind to Aβ, inhibit aggregation, and reduce its toxicity. Previously, we identified strand G of transthyretin as a specific Aβ binding domain. In this work we further explore and define the necessary features of this binding domain. We demonstrate that peptides derived from transthyretin bind Aβ and inhibit its toxicity. We also show that, although both transthyretin and transthyretin-derived peptides bind Aβ and inhibit toxicity, they differ significantly in their effect on Aβ aggregation.
Figures





References
-
- Hardy J.; Selkoe D. J. (2002) Medicine - The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 297, 353–356. - PubMed
-
- Karran E.; Mercken M.; De Strooper B. (2011) The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat. Rev. Drug Discovery 10, 698–U1600. - PubMed
-
- Hawkes C. A.; Ng V.; McLaurin J. (2009) Small molecule inhibitors of Abeta aggregation and neurotoxicity. Drug Dev. Res. 70, 111–124.
-
- Yang F. S.; Lim G. P.; Begum A. N.; Ubeda O. J.; Simmons M. R.; Ambegaokar S. S.; Chen P. P.; Kayed R.; Glabe C. G.; Frautschy S. A.; Cole G. M. (2005) Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 280, 5892–5901. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

