Current trends in West Nile virus vaccine development
- PMID: 24689659
- PMCID: PMC4279923
- DOI: 10.1586/14760584.2014.906309
Current trends in West Nile virus vaccine development
Abstract
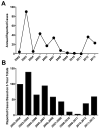
West Nile virus (WNV) is a mosquito-borne flavivirus that has become endemic in the United States. From 1999-2012, there have been 37088 reported cases of WNV and 1549 deaths, resulting in a 4.2% case-fatality rate. Despite development of effective WNV vaccines for horses, there is no vaccine to prevent human WNV infection. Several vaccines have been tested in preclinical studies and to date there have been eight clinical trials, with promising results in terms of safety and induction of antiviral immunity. Although mass vaccination is unlikely to be cost effective, implementation of a targeted vaccine program may be feasible if a safe and effective vaccine can be brought to market. Further evaluation of new and advanced vaccine candidates is strongly encouraged.
Figures


References
-
-
Roehrig JT. West nile virus in the United States - a historical perspective. Viruses. 2013;5(12):3088–3108. • •This review provides a candid, first-hand account and historical perspective on the actions taken to characterize and control WNV after introduction to the United States in 1999
-
-
- Kramer LD, Styer LM, Ebel GD. A global perspective on the epidemiology of West Nile virus. Annu Rev Entomol. 2008;53:61–81. - PubMed
-
-
Lanciotti RS, Roehrig JT, Deubel V, et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286(5448):2333–2337. • Performed full length genomic sequencing of WNV-NY99 and discovered that it was most closely related to an Israeli strain identified in 1998 that also caused avian fatalities
-
Websites
-
- CDC . WNV Statistics & Maps. CDC; [Accessed January 30 2014]. 2014. [Online] Available: http://www.cdc.gov/westnile/statsMaps/
-
- PHAC [Accessed January 19 2014];Maps & Stats - West Nile virus - Public Health Agency of Canada. 2013 Online. Available: http://www.phac-aspc.gc.ca/wnv-vwn/index-eng.php.
-
- EPISOUTH . Network for Communicable Disease Control in Southern Europe and Mediterranean Countries. EpiSouth; [Accessed January 19 2014]. 2014. Online. Available: http://www.episouth.org/home.php.
-
- USGS . USGS West Nile Virus Maps. USGS; [Accessed January 16 2014]. 2014. Online. Available: http://diseasemaps.usgs.gov/wnv_historical.html.
-
- FDA [Accessed January 16 2014];Countering Bioterrorism Questions and Answers. 2012 Online. Available: http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/ProductSecu....
Patent Applications
-
- Coller BA, Pai V, Weeks-Levy C, Ogata S. United States Patent Application No. US20120141520 A1: Recombinant subunit West Nile virus vaccine for protection of human subjects. 2012.
Vaccine Package Inserts/Recall Notices
-
- YF-VAX Package Insert. Aventis Pasteur. Swiftwater, PA: 2005.
-
- IXIARO Package Insert. Intercell-AG. Vienna: 2013.
-
- Whitehead B. Updated recall notice PreveNile® West Nile Vaccine. Intervet Schering-Plough Animal Health; 2010.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
