Redox-neutral α-oxygenation of amines: reaction development and elucidation of the mechanism
- PMID: 24689802
- PMCID: PMC4333595
- DOI: 10.1021/ja501988b
Redox-neutral α-oxygenation of amines: reaction development and elucidation of the mechanism
Abstract
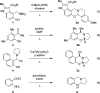
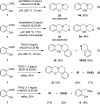
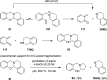
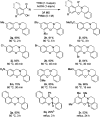
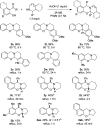
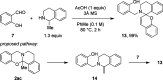
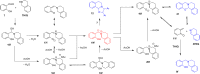
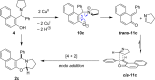
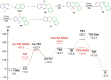
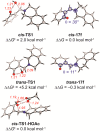
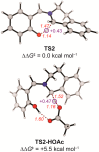
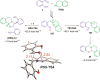
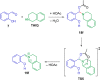
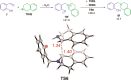
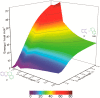
Cyclic secondary amines and 2-hydroxybenzaldehydes or related ketones react to furnish benzo[e][1,3]oxazine structures in generally good yields. This overall redox-neutral amine α-C-H functionalization features a combined reductive N-alkylation/oxidative α-functionalization and is catalyzed by acetic acid. In contrast to previous reports, no external oxidants or metal catalysts are required. Reactions performed under modified conditions lead to an apparent reductive amination and the formation of o-hydroxybenzylamines in a process that involves the oxidation of a second equivalent of amine. A detailed computational study employing density functional theory compares different mechanistic pathways and is used to explain the observed experimental findings. Furthermore, these results also reveal the origin of the catalytic efficiency of acetic acid in these transformations.
Figures
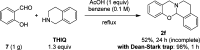


References
-
- Nonato M. G.; Garson M. J.; Truscott R. J. W.; Carver J. A. Phytochemistry (Elsevier) 1993, 34, 1159.
- Langer P.; Frackenpohl J.; Hoffmann H. M. R. J. Chem. Soc., Perkin Trans. 1 1998, 801.
- Heys L.; Moore C. G.; Murphy P. J. Chem. Soc. Rev. 2000, 29, 57.
- Troast D. M.; Porco J. A. Jr. Org. Lett. 2002, 4, 991. - PubMed
- Jiang X.; Garcia-Fortanet J.; De Brabander J. K. J. Am. Chem. Soc. 2005, 127, 11254. - PubMed
- Kock M.; Grube A.; Seiple I. B.; Baran P. S. Angew. Chem., Int. Ed. 2007, 46, 6586. - PubMed
- Miyashita M. Pure Appl. Chem. 2007, 79, 651.
- Sakaguchi K.; Ayabe M.; Watanabe Y.; Okada T.; Kawamura K.; Shiada T.; Ohfune Y. Org. Lett. 2008, 10, 5449. - PubMed
- Su S.; Rodriguez R. A.; Baran P. S. J. Am. Chem. Soc. 2011, 133, 13922. - PMC - PubMed
-
- Leonard N. J.; Leubner G. W. J. Am. Chem. Soc. 1949, 71, 3408.
- Galinovsky F.; Wagner A.; Weiser R. Monatsh. Chem. 1951, 82, 551.
- Shono T.; Matsumura Y.; Tsubata K. J. Am. Chem. Soc. 1981, 103, 1172.
- Miao C. K.; Sorcek R.; Jones P. J. Tetrahedron Lett. 1993, 34, 2259.
- Ishii A.; Higashiama K.; Mikami K. Synlett 1997, 1381.
- Jiang J.; DeVita R. J.; Doss G. A.; Goulet M. T.; Wyvratt M. J. J. Am. Chem. Soc. 1999, 121, 593.
- Ferraris D.; Dudding T.; Young B.; Drury W. J.; Lectka T. J. Org. Chem. 1999, 64, 2168.
- Veerman J. J. N.; Rutjes F. P. J. T.; van Maarseveen J. H.; Hiemstra H. Tetrahedron Lett. 1999, 40, 6079.
- Yamazaki N.; Ito T.; Kibayashi C. Org. Lett. 2000, 2, 465. - PubMed
- Cimarelli C.; Palmieri G.; Volpini E. Tetrahedron 2001, 57, 6089.
- Kinderman S. S.; Doodeman R.; van Beijma J. W.; Russcher J. C.; Tjen K. C. M. F.; Kooistra T. M.; Mohaselzadeh H.; van Maarseveen J. H.; Hiemstra H.; Schoemaker H. E.; Rutjes F. P. J. T. Adv. Synth. Catal. 2002, 344, 736.
- Lu J.; Xu X.; Wang S.; Wang C.; Hu Y.; Hu H. J. Chem. Soc., Perkin Trans. 1 2002, 2900.
- Lebouvier N.; Laroche C.; Huguenot F.; Brigaud T. Tetrahedron Lett. 2002, 43, 2827.
- Gosselin F.; Roy A.; O’Shea P. D.; Chen C.; Volante R. P. Org. Lett. 2004, 6, 641. - PubMed
- Dong Y.; Sun J.; Wang X.; Xu X.; Cao L.; Hu Y. Tetrahedron: Asymmetry 2004, 15, 1667.
- Harayama Y.; Yoshida M.; Kamimura D.; Kita Y. Chem. Commun. 2005, 1764. - PubMed
- Wang X.; Dong Y.; Sun J.; Xu X.; Li R.; Hu Y. J. Org. Chem. 2005, 70, 1897. - PubMed
- Barragan E.; Olivo H. F.; Romero-Ortega M.; Sarduy S. J. Org. Chem. 2005, 70, 4214. - PubMed
- Myers E. L.; de Vries J. G.; Aggarwal V. K. Angew. Chem., Int. Ed. 2007, 46, 1893. - PubMed
- Peterson A. E.; Jacobsen E. N. Angew. Chem., Int. Ed. 2009, 48, 6328. - PMC - PubMed
- Othman R. B.; Affani R.; Tranchant M. J.; Antoniotti S.; Dalla V.; Dunach E. Angew. Chem., Int. Ed. 2010, 49, 776. - PubMed
- Chen G.; Wang X.; Su D.; Liu H.; Liu F.; Hu Y. J. Org. Chem. 2010, 75, 1911. - PubMed
- Peixoto S.; Nguyen T. M.; Crich D.; Delpech B.; Marazano C. Org. Lett. 2010, 12, 4760. - PubMed
- Zhang W.; Dai Y.; Wang X.; Zhang W. Tetrahedron Lett. 2011, 52, 6122.
- Jurberg I. D.; Peng B.; Wostefeld E.; Wasserloos M.; Maulide N. Angew. Chem., Int. Ed. 2012, 51, 1950. - PubMed
- Shi S. L.; Wei X. F.; Shimizu Y.; Kanai M. J. Am. Chem. Soc. 2012, 134, 17019. - PubMed
-
- Chylinska J. B.; Janoiec M.; Urbanski T. Br. J. Pharmacol. 1971, 43, 649. - PMC - PubMed
- Scoda J.; Votruba I.; Farkas J. Biochem. Pharmacol. 1979, 28, 1837. - PubMed
- Latif N.; Mishriky N.; Assad F. M. Aust. J. Chem. 1982, 35, 1037.
- Waisser K.; Gregor J.; Kubicova L.; Klimesova V.; Kunes J.; Machacek M.; Kaustova J. Eur. J. Med. Chem. 2000, 35, 733. - PubMed
- Petrlikova E.; Waisser K.; Divisova H.; Husakova P.; Vrabcova P.; Kunes J.; Kolar K.; Stolarikova J. Bioorg. Med. Chem. 2010, 18, 8178. - PubMed
- Kategaonkar A. H.; Sonar S. S.; Pokalwar R. U.; Kategaonkar A. H.; Shingate B. B.; Shingare M. S. Bull. Korean Chem. Soc. 2010, 31, 1657.
- Mathew B. P.; Kumar A.; Sharma S.; Shukla P. K.; Nath M. Eur. J. Med. Chem. 2010, 45, 1502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

