Abnormal cortical growth in schizophrenia targets normative modules of synchronized development
- PMID: 24690112
- PMCID: PMC4395469
- DOI: 10.1016/j.biopsych.2014.02.010
Abnormal cortical growth in schizophrenia targets normative modules of synchronized development
Abstract
Background: Schizophrenia is a disorder of brain connectivity and altered neurodevelopmental processes. Cross-sectional case-control studies in different age groups have suggested that deficits in cortical thickness in childhood-onset schizophrenia may normalize over time, suggesting a disorder-related difference in cortical growth trajectories.
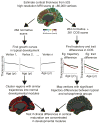
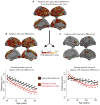
Methods: We acquired magnetic resonance imaging scans repeated over several years for each subject, in a sample of 106 patients with childhood-onset schizophrenia and 102 age-matched healthy volunteers. Using semiparametric regression, we modeled the effect of schizophrenia on the growth curve of cortical thickness in ~80,000 locations across the cortex, in the age range 8 to 30 years. In addition, we derived normative developmental modules composed of cortical regions with similar maturational trajectories for cortical thickness in typical brain development.
Results: We found abnormal nonlinear growth processes in prefrontal and temporal areas that have previously been implicated in schizophrenia, distinguishing for the first time between cortical areas with age-constant deficits in cortical thickness and areas whose maturational trajectories are altered in schizophrenia. In addition, we showed that when the brain is divided into five normative developmental modules, the areas with abnormal cortical growth overlap significantly only with the cingulo-fronto-temporal module.
Conclusions: These findings suggest that abnormal cortical development in schizophrenia may be modularized or constrained by the normal community structure of developmental modules of the human brain connectome.
Keywords: Neuroimaging; penalized splines; psychosis; system; topology.
Copyright © 2014 Society of Biological Psychiatry. All rights reserved.
Figures


Comment in
-
Finding pieces to the puzzle of brain structure in schizophrenia.Biol Psychiatry. 2014 Sep 15;76(6):432-3. doi: 10.1016/j.biopsych.2014.07.003. Biol Psychiatry. 2014. PMID: 25149348 Free PMC article. No abstract available.
References
-
- Nenadic I, Gaser C, Sauer H. Heterogeneity of brain structural variation and the structural imaging endophenotypes in schizophrenia. Neuropsychobiology. 2012;66:44–49. - PubMed
-
- Ellison-Wright I, Bullmore E. Schizophr Res. Vol. 108. Elsevier; 2009. Meta-analysis of diffusion tensor imaging studies in schizophrenia; pp. 3–10. - PubMed
-
- Honea R, Crow TJ, Passingham D, Mackay CE. Am J Psychiatry. Vol. 162. American Psychiatric Association; 2005. Regional Deficits in Brain Volume in Schizophrenia: A Meta-Analysis of Voxel-Based Morphometry Studies; pp. 2233–2245. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

