Development of immortalized mouse aortic endothelial cell lines
- PMID: 24690145
- PMCID: PMC4230636
- DOI: 10.1186/2045-824X-6-7
Development of immortalized mouse aortic endothelial cell lines
Abstract
Background: The understanding of endothelial cell biology has been facilitated by the availability of primary endothelial cell cultures from a variety of sites and species; however, the isolation and maintenance of primary mouse aortic endothelial cells (MAECs) remain a formidable challenge. Culturing MAECs is difficult as they are prone to phenotypic drift during culture. Therefore, there is a need to have a dependable in vitro culture system, wherein the primary endothelial cells retain their properties and phenotypes.
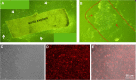
Methods: Here, we developed an effective method to prepare immortalized MAEC (iMAEC) lines. Primary MAECs, initially isolated from aortic explants, were immortalized using a retrovirus expressing polyoma middle T-antigen. Immortalized cells were then incubated with DiI-acetylated-low density lipoprotein and sorted via flow cytometry to isolate iMAECs.
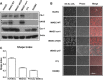
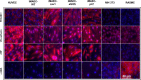
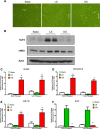
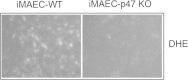
Results: iMAECs expressed common markers of endothelial cells, including PECAM1, eNOS, VE-cadherin, and von Willebrand Factor. iMAECs aligned in the direction of imposed laminar shear and retained the ability to form tubes. Using this method, we have generated iMAEC lines from wild-type and various genetically modified mice such as p47phox-/-, eNOS-/-, and caveolin-1-/-.
Conclusion: In summary, generation of iMAEC lines from various genetically modified mouse lines provides an invaluable tool to study vascular biology and pathophysiology.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

