Optimization of 3-D organotypic primary colonic cultures for organ-on-chip applications
- PMID: 24690469
- PMCID: PMC4022271
- DOI: 10.1186/1754-1611-8-9
Optimization of 3-D organotypic primary colonic cultures for organ-on-chip applications
Abstract
Background: New advances enable long-term organotypic culture of colonic epithelial stem cells that develop into structures known as colonoids. Colonoids represent a primary tissue source acting as a potential starting material for development of an in vitro model of the colon. Key features of colonic crypt isolation and subsequent colonoid culture have not been systematically optimized compromising efficiency and reproducibility. Here murine crypt isolation yield and quality are optimized, and colonoid culture efficiency measured in microfabricated culture devices.
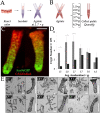
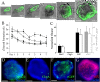
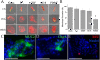
Results: An optimal incubation time of 60 min in a chelating buffer released 280,000 ± 28,000 crypts from the stroma of a single colon with 79.3% remaining intact. Mechanical agitation using an average acceleration of 1.5 × g liberated the highest quality crypts with 86% possessing well-defined lumens. Culture in 50% Matrigel resulted in the highest colonoid formation efficiency of 33 ± 5%. Immunostaining demonstrated that colonoids isolated under these conditions possessed stem/progenitor cells and differentiated cell lineages. Microfabrication substrates (glass, polystyrene, PDMS, and epoxy photoresists: SU-8 and 1002-F) were tested for compatibility with colonoid culture. PDMS promoted formation of 3-D colonoids containing stem/progenitor cells, while other substrates promoted outgrowth of a 2-D epithelial monolayer composed of differentiated cells.
Conclusion: Improved crypt isolation and 3-D colonoid culture, along with an understanding of colonic epithelial cell behavior in the presence of microfabrication substrates will support development of 'organ-on-a-chip' approaches for studies using primary colonic epithelium.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

