Treating infant colic with the probiotic Lactobacillus reuteri: double blind, placebo controlled randomised trial
- PMID: 24690625
- PMCID: PMC3972414
- DOI: 10.1136/bmj.g2107
Treating infant colic with the probiotic Lactobacillus reuteri: double blind, placebo controlled randomised trial
Abstract
Objective: To determine whether the probiotic Lactobacillus reuteri DSM 17938 reduces crying or fussing in a broad community based sample of breastfed infants and formula fed infants with colic aged less than 3 months.
Design: Double blind, placebo controlled randomised trial.
Setting: Community based sample (primary and secondary level care centres) in Melbourne, Australia.
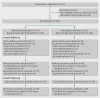
Participants: 167 breastfed infants or formula fed infants aged less than 3 months meeting Wessel's criteria for crying or fussing: 85 were randomised to receive probiotic and 82 to receive placebo.
Interventions: Oral daily L reuteri (1 × 10(8) colony forming units) versus placebo for one month.
Main outcomes measures: The primary outcome was daily duration of cry or fuss at 1 month. Secondary outcomes were duration of cry or fuss; number of cry or fuss episodes; sleep duration of infant at 7, 14, and 21 days, and 1 and 6 months; maternal mental health (Edinburgh postnatal depression subscale); family functioning (paediatric quality of life inventory), parent quality adjusted life years (assessment of quality of life) at 1 and 6 months; infant functioning (paediatric quality of life inventory) at 6 months; infant faecal microbiota (microbial diversity, colonisation with Escherichia coli), and calprotectin levels at 1 month. In intention to treat analyses the two groups were compared using regression models adjusted for potential confounders.
Results: Of 167 infants randomised from August 2011 to August 2012, 127 (76%) were retained to primary outcome; of these, a subset was analysed for faecal microbial diversity, E coli colonisation, and calprotectin levels. Adherence was high. Mean daily cry or fuss time fell steadily in both groups. At 1 month, the probiotic group cried or fussed 49 minutes more than the placebo group (95% confidence interval 8 to 90 minutes, P=0.02); this mainly reflected more fussing, especially for formula fed infants. The groups were similar on all secondary outcomes. No study related adverse events occurred.
Conclusions: L reuteri DSM 17938 did not benefit a community sample of breastfed infants and formula fed infants with colic. These findings differ from previous smaller trials of selected populations and do not support a general recommendation for the use of probiotics to treat colic in infants.
Trial registration: Current Controlled Trials ISRCTN95287767.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures

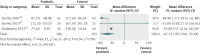

Comment in
-
Probiotics and infant colic.BMJ. 2014 Apr 1;348:g2286. doi: 10.1136/bmj.g2286. BMJ. 2014. PMID: 24690628 No abstract available.
-
Study concludes L. reuteri not effective for infant colic, but findings may be limited by participants' heterogeneity.Evid Based Med. 2014 Dec;19(6):215. doi: 10.1136/ebmed-2014-110027. Epub 2014 Aug 27. Evid Based Med. 2014. PMID: 25165159 No abstract available.
-
Probiotics for colic? A PURL update.J Fam Pract. 2014 Oct;63(10):602. J Fam Pract. 2014. PMID: 25343150 Free PMC article.
References
-
- Wake M, Morton-Allen E, Poulakis Z, Hiscock H, Gallagher S, Oberklaid F. Prevalence, stability, and outcomes of cry-fuss and sleep problems in the first 2 years of life: prospective community-based study. Pediatrics 2006;117:836-42. - PubMed
-
- Wessel MA, Cobb JC, Jackson EB, Harris GS, Detwiler AC. Paroxysmal fussing in infancy, sometimes called “colic.” Pediatrics 1954;14:421-34. - PubMed
-
- McMahon C, Barnett B, Kowalenko N, Tennant C, Don N. Postnatal depression, anxiety and unsettled infant behaviour. Aust N Z J Psychiatry 2001;35:581-8. - PubMed
-
- Howard CR, Lanphear N, Lanphear BP, Eberly S, Lawrence RA. Parental responses to infant crying and colic: the effect on breastfeeding duration. Breastfeed Med 2006;1:146-55. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
