Both structural and non-structural forms of the readthrough protein of cucurbit aphid-borne yellows virus are essential for efficient systemic infection of plants
- PMID: 24691251
- PMCID: PMC3972232
- DOI: 10.1371/journal.pone.0093448
Both structural and non-structural forms of the readthrough protein of cucurbit aphid-borne yellows virus are essential for efficient systemic infection of plants
Abstract
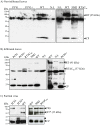
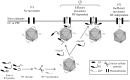
Cucurbit aphid-borne yellows virus (CABYV) is a polerovirus (Luteoviridae family) with a capsid composed of the major coat protein and a minor component referred to as the readthrough protein (RT). Two forms of the RT were reported: a full-length protein of 74 kDa detected in infected plants and a truncated form of 55 kDa (RT*) incorporated into virions. Both forms were detected in CABYV-infected plants. To clarify the specific roles of each protein in the viral cycle, we generated by deletion a polerovirus mutant able to synthesize only the RT* which is incorporated into the particle. This mutant was unable to move systemically from inoculated leaves inferring that the C-terminal half of the RT is required for efficient long-distance transport of CABYV. Among a collection of CABYV mutants bearing point mutations in the central domain of the RT, we obtained a mutant impaired in the correct processing of the RT which does not produce the RT*. This mutant accumulated very poorly in upper non-inoculated leaves, suggesting that the RT* has a functional role in long-distance movement of CABYV. Taken together, these results infer that both RT proteins are required for an efficient CABYV movement.
Conflict of interest statement
Figures



Similar articles
-
Incidence and molecular diversity of poleroviruses infecting cucurbit crops and weed plants in Thailand.Arch Virol. 2017 Jul;162(7):2083-2090. doi: 10.1007/s00705-017-3332-2. Epub 2017 Mar 28. Arch Virol. 2017. PMID: 28352973
-
First Report of Cucurbit aphid-borne yellows virus in Tunisia Causing Yellows on Five Cucurbitacious Species.Plant Dis. 2005 Jul;89(7):776. doi: 10.1094/PD-89-0776B. Plant Dis. 2005. PMID: 30791260
-
First Report of Cucurbit aphid-borne yellows virus in Iran Causing Yellows on Four Cucurbit Crops.Plant Dis. 2006 Apr;90(4):526. doi: 10.1094/PD-90-0526A. Plant Dis. 2006. PMID: 30786614
-
A Stem-Loop Structure in Potato Leafroll Virus Open Reading Frame 5 (ORF5) Is Essential for Readthrough Translation of the Coat Protein ORF Stop Codon 700 Bases Upstream.J Virol. 2018 May 14;92(11):e01544-17. doi: 10.1128/JVI.01544-17. Print 2018 Jun 1. J Virol. 2018. PMID: 29514911 Free PMC article.
-
Construction of an infectious full-length cDNA clone of a recombinant isolate of cucurbit aphid-borne yellows virus from Brazil.Virus Genes. 2023 Feb;59(1):163-166. doi: 10.1007/s11262-022-01948-y. Epub 2022 Oct 28. Virus Genes. 2023. PMID: 36306006
Cited by
-
Polerovirus genomic variation.Virus Evol. 2021 Dec 4;7(2):veab102. doi: 10.1093/ve/veab102. eCollection 2021 Sep. Virus Evol. 2021. PMID: 35299789 Free PMC article.
-
Visualization of Host-Polerovirus Interaction Topologies Using Protein Interaction Reporter Technology.J Virol. 2015 Dec 9;90(4):1973-87. doi: 10.1128/JVI.01706-15. Print 2016 Feb 15. J Virol. 2015. PMID: 26656710 Free PMC article.
-
Arabidopsis RNA-binding proteins interact with viral structural proteins and modify turnip yellows virus accumulation.Plant Physiol. 2024 Dec 23;197(1):kiae590. doi: 10.1093/plphys/kiae590. Plant Physiol. 2024. PMID: 39658301 Free PMC article.
-
Molecular Characterization of a Novel Enamovirus Infecting Raspberry.Viruses. 2023 Nov 21;15(12):2281. doi: 10.3390/v15122281. Viruses. 2023. PMID: 38140523 Free PMC article.
-
Evaluation of a Bead-Free Coimmunoprecipitation Technique for Identification of Virus-Host Protein Interactions Using High-Resolution Mass Spectrometry.J Biomol Tech. 2017 Sep;28(3):111-121. doi: 10.7171/jbt.17-2803-002. Epub 2017 Jul 24. J Biomol Tech. 2017. PMID: 28785175 Free PMC article.
References
-
- Guilley H, Wipf-Scheibel C, Richards K, Lecoq H, Jonard G (1994) Nucleotide sequence of cucurbit aphid-borne yellows luteovirus. Virology 202: 1012–1017. - PubMed
-
- Brault V, Uzest M, Monsion B, Jacquot E, Blanc S (2010) Aphids as transport devices for plant viruses. C R Biol 333: 524–538. - PubMed
-
- Esau K, Hoefert LL (1972) Development of infection with beet western yellows luteovirus in the sugarbeet. Virology 48: 724–738. - PubMed
-
- Shepardson S, Esau K, McCrum R (1980) Ultrastructure of potato leaf phloem infected with potato leafroll virus. Virology 105: 379–392. - PubMed
-
- Mutterer JD, Stussi-Garaud C, Michler P, Richards KE, Jonard G, et al. (1999) Role of the beet western yellows virus readthrough protein in virus movement in Nicotiana clevelandii. J Gen Virol 80: 2771–2778. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

