Senescence-associated SIN3B promotes inflammation and pancreatic cancer progression
- PMID: 24691445
- PMCID: PMC4001548
- DOI: 10.1172/JCI72619
Senescence-associated SIN3B promotes inflammation and pancreatic cancer progression
Abstract
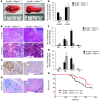
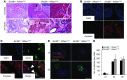
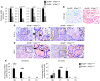
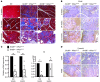
Pancreatic ductal adenocarcinoma (PDAC) is strikingly resistant to conventional therapeutic approaches. We previously demonstrated that the histone deacetylase-associated protein SIN3B is essential for oncogene-induced senescence in cultured cells. Here, using a mouse model of pancreatic cancer, we have demonstrated that SIN3B is required for activated KRAS-induced senescence in vivo. Surprisingly, impaired senescence as the result of genetic inactivation of Sin3B was associated with delayed PDAC progression and correlated with an impaired inflammatory response. In murine and human pancreatic cells and tissues, levels of SIN3B correlated with KRAS-induced production of IL-1α. Furthermore, evaluation of human pancreatic tissue and cancer cells revealed that Sin3B was decreased in control and PDAC samples, compared with samples from patients with pancreatic inflammation. These results indicate that senescence-associated inflammation positively correlates with PDAC progression and suggest that SIN3B has potential as a therapeutic target for inhibiting inflammation-driven tumorigenesis.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

