Harnessing FOXP3+ regulatory T cells for transplantation tolerance
- PMID: 24691478
- PMCID: PMC3973097
- DOI: 10.1172/JCI67226
Harnessing FOXP3+ regulatory T cells for transplantation tolerance
Abstract
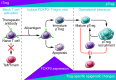
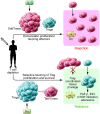
Early demonstrations that mice could be tolerized to transplanted tissues with short courses of immunosuppressive therapy and that with regard to tolerance to self, CD4+FOXP3+ regulatory T cells (Tregs) appeared to play a critical role, have catalyzed strategies to harness FOXP3-dependent processes to control rejection in human transplantation. This review seeks to examine the scientific underpinning for this new approach to finesse immunosuppression.
Figures
References
-
- Benjamin RJ, Waldmann H.Induction of tolerance by monoclonal antibody therapy. Nature. 198632060614z 49–451 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

