Structure-function relationships of ErbB RTKs in the plasma membrane of living cells
- PMID: 24691959
- PMCID: PMC3970415
- DOI: 10.1101/cshperspect.a008961
Structure-function relationships of ErbB RTKs in the plasma membrane of living cells
Abstract
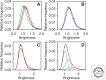
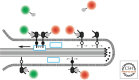
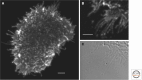
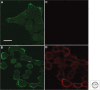
We review the states of the ErbB family of receptor tyrosine kinases (RTKs), primarily the EGF receptor (EGFR, ErbB1, HER1) and the orphan receptor ErbB2 as they exist in living mammalian cells, focusing on four main aspects: (1) aggregation state and distribution in the plasma membrane; (2) conformational features of the receptors situated in the plasma membrane, compared to the crystallographic structures of the isolated extracellular domains; (3) coupling of receptor disposition on filopodia with the transduction of signaling ligand gradients; and (4) ligand-independent receptor activation by application of a magnetic field.
Figures

References
-
- Alexi X, Berditchevski F, Odintsova E 2011. The effect of cell-ECM adhesion on signalling via the ErbB family of growth factor receptors. Biochem Soc Trans 39: 568–573 - PubMed
-
- Berger MB, Mendrola JM, Lemmon MA 2004. ErbB3/HER3 does not homodimerize upon neuregulin binding at the cell surface. FEBS Lett 569: 332–336 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
