Erasers of histone acetylation: the histone deacetylase enzymes
- PMID: 24691964
- PMCID: PMC3970420
- DOI: 10.1101/cshperspect.a018713
Erasers of histone acetylation: the histone deacetylase enzymes
Abstract
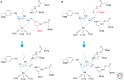
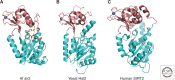
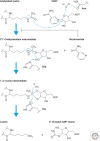
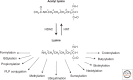
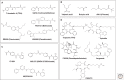
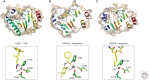
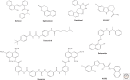
Histone deacetylases (HDACs) are enzymes that catalyze the removal of acetyl functional groups from the lysine residues of both histone and nonhistone proteins. In humans, there are 18 HDAC enzymes that use either zinc- or NAD(+)-dependent mechanisms to deacetylate acetyl lysine substrates. Although removal of histone acetyl epigenetic modification by HDACs regulates chromatin structure and transcription, deacetylation of nonhistones controls diverse cellular processes. HDAC inhibitors are already known potential anticancer agents and show promise for the treatment of many diseases.
Figures



References
-
- Allis D, Jenuwein T, Reinberg D 2014. Overview and concepts. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a018739 - DOI
-
- Asaba T, Suzuki T, Ueda R, Tsumoto H, Nakagawa H, Miyata N 2009. Inhibition of human sirtuins by in situ generation of an acetylated lysine-ADP-ribose conjugate. J Am Chem Soc 131: 6989–6996 - PubMed
-
- Avalos JL, Boeke JD, Wolberger C 2004. Structural basis for the mechanism and regulation of Sir2 enzymes. Mol Cell 13: 639–648 - PubMed
-
- Ayer DE 1999. Histone deacetylases: Transcriptional repression with SINers and NuRDs. Trends Cell Biol 9: 193–198 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
