The EGFR family: not so prototypical receptor tyrosine kinases
- PMID: 24691965
- PMCID: PMC3970421
- DOI: 10.1101/cshperspect.a020768
The EGFR family: not so prototypical receptor tyrosine kinases
Abstract
The epidermal growth factor receptor (EGFR) was among the first receptor tyrosine kinases (RTKs) for which ligand binding was studied and for which the importance of ligand-induced dimerization was established. As a result, EGFR and its relatives have frequently been termed "prototypical" RTKs. Many years of mechanistic studies, however, have revealed that--far from being prototypical--the EGFR family is quite unique. As we discuss in this review, the EGFR family uses a distinctive "receptor-mediated" dimerization mechanism, with ligand binding inducing a dramatic conformational change that exposes a dimerization arm. Intracellular kinase domain regulation in this family is also unique, being driven by allosteric changes induced by asymmetric dimer formation rather than the more typical activation-loop phosphorylation. EGFR family members also distinguish themselves from other RTKs in having an intracellular juxtamembrane (JM) domain that activates (rather than autoinhibits) the receptor and a very large carboxy-terminal tail that contains autophosphorylation sites and serves an autoregulatory function. We discuss recent advances in mechanistic aspects of all of these components of EGFR family members, attempting to integrate them into a view of how RTKs in this important class are regulated at the cell surface.
Figures

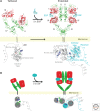



References
-
- Alvarez CV, Shon KJ, Miloso M, Beguinot L 1995. Structural requirements of the epidermal growth factor receptor for tyrosine phosphorylation of eps8 and eps15, substrates lacking Src SH2 homology domains. J Biol Chem 270: 16271–16276 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
