Quantitative protein analysis using enzymatic [¹⁸O]water labeling
- PMID: 24692014
- PMCID: PMC4066220
- DOI: 10.1002/0471140864.ps2304s76
Quantitative protein analysis using enzymatic [¹⁸O]water labeling
Abstract
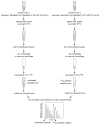
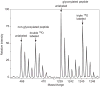
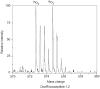
This unit describes the stepwise procedure for differential oxygen isotope labeling of peptides and mass spectrometric quantification of relative protein levels in comparative proteomic experiments. The [¹⁸O] labeling of peptides happens at the peptide C-terminus and is achieved via the enzymatic oxygen exchange of tryptic peptides via catalysis of immobilized trypsin. Experimental considerations in effective incorporation and stabilization of the oxygen labels are discussed. Methods for mass spectrometric quantification of peptides with differential [¹⁶O] and [¹⁸O] isotopes are presented.
Keywords: 18O-labeling; back-exchange; enzymatic oxygen labeling; immobilized trypsin; quantitative proteomics.
Copyright © 2014 John Wiley & Sons, Inc.
Figures


References
-
- Fenselau C, Yao X. 18O2-Labeling in quantitative proteomic strategies: a status report. J Proteome Res. 2009;8:2140–2143. - PubMed
-
- Jiang H, Ramos AA, Yao X. Targeted quantitation of overexpressed and endogenous cystic fibrosis transmembrane conductance regulator using multiple reaction monitoring tandem mass spectrometry and oxygen stable isotope dilution. Anal Chem. 2010;82:336–342. - PubMed
-
- Reynolds KJ, Yao X, Fenselau C. Proteolytic 18O labeling for comparative proteomics: evaluation of endoprotease Glu-C as the catalytic agent. J Proteome Res. 2002;1:27–33. - PubMed
-
- Schnolzer M, Jedrzejewski P, Lehmann WD. Protease-catalyzed incorporation of 18O into peptide fragments and its application for protein sequencing by electrospray and matrix-assisted laser desorption/ionization mass spectrometry. Electrophoresis. 1996;17:945–953. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

