Energy metabolism in the liver
- PMID: 24692138
- PMCID: PMC4050641
- DOI: 10.1002/cphy.c130024
Energy metabolism in the liver
Abstract
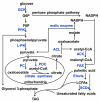
The liver is an essential metabolic organ, and its metabolic function is controlled by insulin and other metabolic hormones. Glucose is converted into pyruvate through glycolysis in the cytoplasm, and pyruvate is subsequently oxidized in the mitochondria to generate ATP through the TCA cycle and oxidative phosphorylation. In the fed state, glycolytic products are used to synthesize fatty acids through de novo lipogenesis. Long-chain fatty acids are incorporated into triacylglycerol, phospholipids, and/or cholesterol esters in hepatocytes. These complex lipids are stored in lipid droplets and membrane structures, or secreted into the circulation as very low-density lipoprotein particles. In the fasted state, the liver secretes glucose through both glycogenolysis and gluconeogenesis. During pronged fasting, hepatic gluconeogenesis is the primary source for endogenous glucose production. Fasting also promotes lipolysis in adipose tissue, resulting in release of nonesterified fatty acids which are converted into ketone bodies in hepatic mitochondria though β-oxidation and ketogenesis. Ketone bodies provide a metabolic fuel for extrahepatic tissues. Liver energy metabolism is tightly regulated by neuronal and hormonal signals. The sympathetic system stimulates, whereas the parasympathetic system suppresses, hepatic gluconeogenesis. Insulin stimulates glycolysis and lipogenesis but suppresses gluconeogenesis, and glucagon counteracts insulin action. Numerous transcription factors and coactivators, including CREB, FOXO1, ChREBP, SREBP, PGC-1α, and CRTC2, control the expression of the enzymes which catalyze key steps of metabolic pathways, thus controlling liver energy metabolism. Aberrant energy metabolism in the liver promotes insulin resistance, diabetes, and nonalcoholic fatty liver diseases.
© 2014 American Physiological Society.
Figures


References
-
- Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616. - PubMed
-
- Agius L. Glucokinase and molecular aspects of liver glycogen metabolism. Biochem J. 2008;414:1–18. - PubMed
-
- Andreelli F, Foretz M, Knauf C, Cani PD, Perrin C, Iglesias MA, Pillot B, Bado A, Tronche F, Mithieux G, Vaulont S, Burcelin R, Viollet B. Liver adenosine monophosphate-activated kinase-alpha2 catalytic subunit is a key target for the control of hepatic glucose production by adiponectin and leptin but not insulin. Endocrinology. 2006;147:2432–2441. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

