Stereocontrolled synthesis of syn-β-Hydroxy-α-amino acids by direct aldolization of pseudoephenamine glycinamide
- PMID: 24692320
- PMCID: PMC4191905
- DOI: 10.1002/anie.201400928
Stereocontrolled synthesis of syn-β-Hydroxy-α-amino acids by direct aldolization of pseudoephenamine glycinamide
Abstract
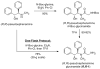
β-Hydroxy-α-amino acids figure prominently as chiral building blocks in chemical synthesis and serve as precursors to numerous important medicines. Reported herein is a method for the synthesis of β-hydroxy-α-amino acid derivatives by aldolization of pseudoephenamine glycinamide, which can be prepared from pseudoephenamine in a one-flask protocol. Enolization of (R,R)- or (S,S)-pseudoephenamine glycinamide with lithium hexamethyldisilazide in the presence of LiCl followed by addition of an aldehyde or ketone substrate affords aldol addition products that are stereochemically homologous with L- or D-threonine, respectively. These products, which are typically solids, can be obtained in stereoisomerically pure form in yields of 55-98 %, and are readily transformed into β-hydroxy-α-amino acids by mild hydrolysis or into 2-amino-1,3-diols by reduction with sodium borohydride. This new chemistry greatly facilitates the construction of novel antibiotics of several different classes.
Keywords: aldol reaction; amino acids; asymmetric synthesis; chiral auxiliaries; synthetic methods.
© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
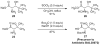
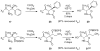

References
-
-
Aldol addition reactions of pseudoephedrine amides (but not glycine amides) are well precedented. See: Vicario JL, Badía D, Domínguez E, Rodríguez M, Carrillo L. J Org Chem. 2000;65:3754–3760.Vicario JL, Rodríguez M, Badía D, Carrillo L, Reyes E. Org Lett. 2004;6:3171–3174.Rodríguez M, Vicario JL, Badía D, Carrillo L. Org Biomol Chem. 2005;3:2026–2030.Ocejo M, Carrillo L, Vicario JS, Badía D, Reyes E. J Org Chem. 2011;76:460–470.Kusuma BR, Brandt GEL, Blagg BSJ. Org Lett. 2012;14:6242–6245.
-
-
-
For practical, scalable syntheses of (R,R)- and (S,S)-pseudoephenamine, see: Mellem KT, Myers AG. Org Lett. 2013;15:5594–5597.; Organic letters; For the alkylation of pseudoephenamine amides, see: Morales MR, Mellem KT, Myers AG. Angew Chem Int Ed. 2012;51:4568–4571.Angew Chem. 2012;124:4646–4649.; For the synthesis and alkylation of pseudoephenamine alaninamide pivaldimine, see: Hugelshofer CL, Mellem KT, Myers AG. Org Lett. 2013;15:3134–3137.
-
-
- Myers AG, Gleason JL, Yoon T. J Am Chem Soc. 1995;117:8488–8489.
- Myers AG, Gleason JL, Yoon T, Kung DW. J Am Chem Soc. 1997;119:656–673.
- Myers AG, Schnider P, Kwon S, Kung DW. J Org Chem. 1999;64:3322–3327. - PubMed
-
-
Lithium chloride was essential to achieve high diastereoselectivities in aldol addition reactions. For example, addition of the lithium enolate derived from 1 (in absence of LiCl) to benzaldehyde afforded a much reduced dr (53% desired: 47% sum of minor isomers).
-
-
-
Pseudoephenamine glycinamide (1) can also be used as the limiting reagent, with a moderate decrease in yield: aldolization of 1 (1.0 equiv) with benzaldehyde (1.2 equiv) provided pure 7 in 65% yield (standard conditions provided the product in 80% yield).
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

