Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration
- PMID: 24692354
- PMCID: PMC4044301
- DOI: 10.1152/physrev.00033.2013
Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration
Abstract
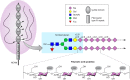
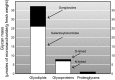
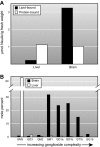
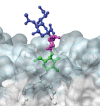
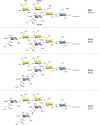
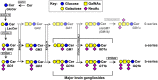
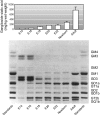
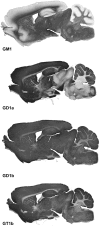
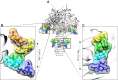
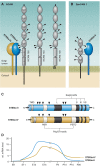
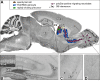
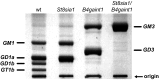
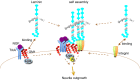
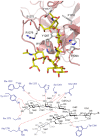
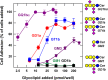
Every cell in nature carries a rich surface coat of glycans, its glycocalyx, which constitutes the cell's interface with its environment. In eukaryotes, the glycocalyx is composed of glycolipids, glycoproteins, and proteoglycans, the compositions of which vary among different tissues and cell types. Many of the linear and branched glycans on cell surface glycoproteins and glycolipids of vertebrates are terminated with sialic acids, nine-carbon sugars with a carboxylic acid, a glycerol side-chain, and an N-acyl group that, along with their display at the outmost end of cell surface glycans, provide for varied molecular interactions. Among their functions, sialic acids regulate cell-cell interactions, modulate the activities of their glycoprotein and glycolipid scaffolds as well as other cell surface molecules, and are receptors for pathogens and toxins. In the brain, two families of sialoglycans are of particular interest: gangliosides and polysialic acid. Gangliosides, sialylated glycosphingolipids, are the most abundant sialoglycans of nerve cells. Mouse genetic studies and human disorders of ganglioside metabolism implicate gangliosides in axon-myelin interactions, axon stability, axon regeneration, and the modulation of nerve cell excitability. Polysialic acid is a unique homopolymer that reaches >90 sialic acid residues attached to select glycoproteins, especially the neural cell adhesion molecule in the brain. Molecular, cellular, and genetic studies implicate polysialic acid in the control of cell-cell and cell-matrix interactions, intermolecular interactions at cell surfaces, and interactions with other molecules in the cellular environment. Polysialic acid is essential for appropriate brain development, and polymorphisms in the human genes responsible for polysialic acid biosynthesis are associated with psychiatric disorders including schizophrenia, autism, and bipolar disorder. Polysialic acid also appears to play a role in adult brain plasticity, including regeneration. Together, vertebrate brain sialoglycans are key regulatory components that contribute to proper development, maintenance, and health of the nervous system.
Figures








References
-
- Abo-Ouf H, Hooper AW, White EJ, Janse van Rensburg HJ, Trigatti BL, Igdoura SA. Deletion of tumor necrosis factor-alpha ameliorates neurodegeneration in Sandhoff disease mice. Hum Mol Genet 22: 3960–3975, 2013 - PubMed
-
- Abrous DN, Montaron MF, Petry KG, Rougon G, Darnaudery M, Le Moal M, Mayo W. Decrease in highly polysialylated neuronal cell adhesion molecules and in spatial learning during ageing are not correlated. Brain Res 744: 285–292, 1997 - PubMed
-
- Albach C, Damoc E, Denzinger T, Schachner M, Przybylski M, Schmitz B. Identification of N-glycosylation sites of the murine neural cell adhesion molecule NCAM by MALDI-TOF and MALDI-FTICR mass spectrometry. Anal Bioanal Chem 378: 1129–1135, 2004 - PubMed
-
- Alhadeff JA, Holzinger RT. Sialyltransferase, sialic acid and sialoglycoconjugates in metastatic tumor and human liver tissue. Int J Biochem 14: 119–126, 1982 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

