Intramolecular interactions that induce helical rearrangement upon rhodopsin activation: light-induced structural changes in metarhodopsin IIa probed by cysteine S-H stretching vibrations
- PMID: 24692562
- PMCID: PMC4022853
- DOI: 10.1074/jbc.M113.527606
Intramolecular interactions that induce helical rearrangement upon rhodopsin activation: light-induced structural changes in metarhodopsin IIa probed by cysteine S-H stretching vibrations
Abstract
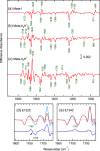
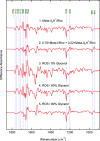
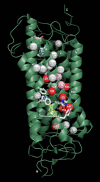
Rhodopsin undergoes rearrangements of its transmembrane helices after photon absorption to transfer a light signal to the G-protein transducin. To investigate the mechanism by which rhodopsin adopts the transducin-activating conformation, the local environmental changes in the transmembrane region were probed using the cysteine S-H group, whose stretching frequency is well isolated from the other protein vibrational modes. The S-H stretching modes of cysteine residues introduced into Helix III, which contains several key residues for the helical movements, and of native cysteine residues were measured by Fourier transform infrared spectroscopy. This method was applied to metarhodopsin IIa, a precursor of the transducin-activating state in which the intramolecular interactions are likely to produce a state ready for helical movements. No environmental change was observed near the ionic lock between Arg-135 in Helix III and Glu-247 in Helix VI that maintains the inactive conformation. Rather, the cysteine residues that showed environmental changes were located around the chromophore, Ala-164, His-211, and Phe-261. These findings imply that the hydrogen bond between Helix III and Helix V involving Glu-122 and His-211 and the hydrophobic packing between Helix III and Helix VI involving Gly-121, Leu-125, Phe-261, and Trp-265 are altered before the helical rearrangement leading toward the active conformation.
Keywords: Fourier Transform IR (FTIR); G-protein-coupled Receptor (GPCR); Phototransduction; Protein Conformation; Rhodopsin; Vision.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




Similar articles
-
Retinal dynamics during light activation of rhodopsin revealed by solid-state NMR spectroscopy.Biochim Biophys Acta. 2010 Feb;1798(2):177-93. doi: 10.1016/j.bbamem.2009.08.013. Epub 2009 Aug 28. Biochim Biophys Acta. 2010. PMID: 19716801 Free PMC article. Review.
-
Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin.Science. 1996 Nov 1;274(5288):768-70. doi: 10.1126/science.274.5288.768. Science. 1996. PMID: 8864113
-
The roles of transmembrane domain helix-III during rhodopsin photoactivation.PLoS One. 2011 Feb 25;6(2):e17398. doi: 10.1371/journal.pone.0017398. PLoS One. 2011. PMID: 21364764 Free PMC article.
-
Transducin-dependent protonation of glutamic acid 134 in rhodopsin.Biochemistry. 2000 Aug 29;39(34):10607-12. doi: 10.1021/bi000912d. Biochemistry. 2000. PMID: 10956053
-
Fourier transform IR spectroscopy study for new insights into molecular properties and activation mechanisms of visual pigment rhodopsin.Biopolymers. 2003;72(3):133-48. doi: 10.1002/bip.10407. Biopolymers. 2003. PMID: 12722110 Review.
Cited by
-
Early Proton Transfer Reaction in a Primate Blue-Sensitive Visual Pigment.Biochemistry. 2022 Dec 6;61(23):2698-2708. doi: 10.1021/acs.biochem.2c00483. Epub 2022 Nov 18. Biochemistry. 2022. PMID: 36399519 Free PMC article.
-
Deprotonation of retinal Schiff base and structural dynamics in the early photoreaction of primate blue cone visual pigment.Biophys J. 2025 Jun 17;124(12):2070-2081. doi: 10.1016/j.bpj.2025.05.004. Epub 2025 May 7. Biophys J. 2025. PMID: 40340252 Free PMC article.
References
-
- Palczewski K., Kumasaka T., Hori T., Behnke C. A., Motoshima H., Fox B. A., Le Trong I., Teller D. C., Okada T., Stenkamp R. E., Yamamoto M., Miyano M. (2000) Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289, 739–745 - PubMed
-
- Rasmussen S. G., Choi H. J., Rosenbaum D. M., Kobilka T. S., Thian F. S., Edwards P. C., Burghammer M., Ratnala V. R., Sanishvili R., Fischetti R. F., Schertler G. F., Weis W. I., Kobilka B. K. (2007) Crystal structure of the human β2 adrenergic G-protein-coupled receptor. Nature 450, 383–387 - PubMed
-
- Farrens D. L., Altenbach C., Yang K., Hubbell W. L., Khorana H. G. (1996) Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science 274, 768–770 - PubMed
-
- Sheikh S. P., Zvyaga T. A., Lichtarge O., Sakmar T. P., Bourne H. R. (1996) Rhodopsin activation blocked by metal-ion-binding sites linking transmembrane helices C and F. Nature 383, 347–350 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

