Investigation of characteristics of feather follicle stem cells and their regeneration potential
- PMID: 24693173
- PMCID: PMC3908268
- DOI: 10.46582/jsrm.0702011
Investigation of characteristics of feather follicle stem cells and their regeneration potential
Abstract
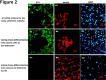
Feather follicles have the extraordinary ability to regenerate and undergo molting cycles. Being tissue-specific stem cells, feather follicle stem cells (FFSCs) have a strong capacity for proliferation and are presumed to be progenitor cells for various epidermal organs. In order to characterize FFSCs and to understand how the feather epidermis and FFSCs produce such a reliable differentiation program resulting in the formation of complex feathers, We developed a culture scheme to select and expand FFSCs from chick feather follicles. FFSCs were examined with cell profiles, mutilpotential differentiation and immunocytochemical staining. FFSCs from a single clone were capable of self-renewal and proliferation. These cells expressed integrin β1, CD49c, cytokeratin 15 (K15), cytokeratin 19 (K19) and a neural-genic cell marker, nestin, but not a teminal differentiation-related keratinocyte marker, cytokeratin 10 (K10). FFSCs could trans-differentiate into adipocytes, neurocytes and keratinocytes. The formation of micro-feather like structures ex-vivo also revealed the potential of regeneration. These results demonstrate that FFSCs possess the properties of stem/progenitor cells and may therefore serve as a useful model for studying mechanisms of stem cell differentiation and their involvement in organ regeneration.
Keywords: Feather follicle stem cells; regeneration; trans-differentiation.
Figures


References
-
- Roger HS, Loren WK. Avian skin development and the evolutionary origin of feathers. J Exp Zool. 2003; 298B: 57–72 - PubMed
-
- Xi Y, Nada Y, Soh T, Fujihara N, Hattori MA. Establishment of Feather Follicle Stem Cells as Potential Vehicles for Delivering Exogenous Genes in Birds. J Rep Dev. 2003; 49: 213-219 - PubMed
-
- Alibardi L. Cell organization of barb ridges in regenerating feathers of the quail: implications of the elongation of barb ridges for the evolution and diversification of feathers. Acta Zool. 2007; 88:101-117
-
- Alibardi L. Keratinization and lipogenesis in epidermal derivatives of the zebrafinch Taenatiopigia castanotis guttata (Ploecidae, Passeriformes, Aves) during embryonic development. J Morphol. 2002; 251: 294-308 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
