Exenatide induces aortic vasodilation increasing hydrogen sulphide, carbon monoxide and nitric oxide production
- PMID: 24693878
- PMCID: PMC3976540
- DOI: 10.1186/1475-2840-13-69
Exenatide induces aortic vasodilation increasing hydrogen sulphide, carbon monoxide and nitric oxide production
Abstract
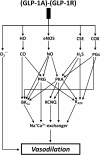
Background: It has been reported that GLP-1 agonist exenatide (exendin-4) decreases blood pressure. The dose-dependent vasodilator effect of exendin-4 has previously been demonstrated, although the precise mechanism is not thoroughly described. Here we have aimed to provide in vitro evidence for the hypothesis that exenatide may decrease central (aortic) blood pressure involving three gasotransmitters, namely nitric oxide (NO) carbon monoxide (CO), and hydrogen sulphide (H2S).
Methods: We determined the vasoactive effect of exenatide on isolated thoracic aortic rings of adult rats. Two millimetre-long vessel segments were placed in a wire myograph and preincubated with inhibitors of the enzymes producing the three gasotransmitters, with inhibitors of reactive oxygen species formation, prostaglandin synthesis, inhibitors of protein kinases, potassium channels or with an inhibitor of the Na+/Ca2+-exchanger.
Results: Exenatide caused dose-dependent relaxation of rat thoracic aorta, which was evoked via the GLP-1 receptor and was mediated mainly by H2S but also by NO and CO. Prostaglandins and superoxide free radical also play a part in the relaxation. Inhibition of soluble guanylyl cyclase significantly diminished vasorelaxation. We found that ATP-sensitive-, voltage-gated- and calcium-activated large-conductance potassium channels are also involved in the vasodilation, but that seemingly the inhibition of the KCNQ-type voltage-gated potassium channels resulted in the most remarkable decrease in the rate of vasorelaxation. Inhibition of the Na+/Ca2+-exchanger abolished most of the vasodilation.
Conclusions: Exenatide induces vasodilation in rat thoracic aorta with the contribution of all three gasotransmitters. We provide in vitro evidence for the potential ability of exenatide to lower central (aortic) blood pressure, which could have relevant clinical importance.
Figures
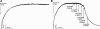
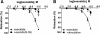

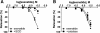
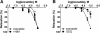

References
-
- Ban K, Noyan-Ashraf MH, Hoefer J, Bolz S-S, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor–dependent and –independent pathways. Circulation. 2008;117:2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. - DOI - PubMed
-
- Richter G, Feddersen O, Wagner U, Barth P, Göke B. GLP-1 stimulates secretion of macromolecules from airways and relaxes pulmonary artery. Am J Physiol. 1993;265:L374–L381. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

