Galactosyltransferases from Arabidopsis thaliana in the biosynthesis of type II arabinogalactan: molecular interaction enhances enzyme activity
- PMID: 24693939
- PMCID: PMC4234293
- DOI: 10.1186/1471-2229-14-90
Galactosyltransferases from Arabidopsis thaliana in the biosynthesis of type II arabinogalactan: molecular interaction enhances enzyme activity
Abstract
Background: Arabinogalactan proteins are abundant proteoglycans present on cell surfaces of plants and involved in many cellular processes, including somatic embryogenesis, cell-cell communication and cell elongation. Arabinogalactan proteins consist mainly of glycan, which is synthesized by post-translational modification of proteins in the secretory pathway. Importance of the variations in the glycan moiety of arabinogalactan proteins for their functions has been implicated, but its biosynthetic process is poorly understood.
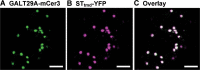
Results: We have identified a novel enzyme in the biosynthesis of the glycan moiety of arabinogalactan proteins. The At1g08280 (AtGALT29A) from Arabidopsis thaliana encodes a putative glycosyltransferase (GT), which belongs to the Carbohydrate Active Enzyme family GT29. AtGALT29A co-expresses with other arabinogalactan GTs, AtGALT31A and AtGLCAT14A. The recombinant AtGALT29A expressed in Nicotiana benthamiana demonstrated a galactosyltransferase activity, transferring galactose from UDP-galactose to a mixture of various oligosaccharides derived from arabinogalactan proteins. The galactose-incorporated products were analyzed using structure-specific hydrolases indicating that the recombinant AtGALT29A possesses β-1,6-galactosyltransferase activity, elongating β-1,6-galactan side chains and forming 6-Gal branches on the β-1,3-galactan main chain of arabinogalactan proteins. The fluorescence tagged AtGALT29A expressed in N. benthamiana was localized to Golgi stacks where it interacted with AtGALT31A as indicated by Förster resonance energy transfer. Biochemically, the enzyme complex containing AtGALT31A and AtGALT29A could be co-immunoprecipitated and the isolated protein complex exhibited increased level of β-1,6-galactosyltransferase activities compared to AtGALT29A alone.
Conclusions: AtGALT29A is a β-1,6-galactosyltransferase and can interact with AtGALT31A. The complex can work cooperatively to enhance the activities of adding galactose residues 6-linked to β-1,6-galactan and to β-1,3-galactan. The results provide new knowledge of the glycosylation process of arabinogalactan proteins and the functional significance of protein-protein interactions among O-glycosylation enzymes.
Figures






References
-
- Seifert GJ, Roberts K. Annual Review of Plant Biology, Volume 58. Palo Alto: Annual Reviews; 2007. The biology of arabinogalactan proteins; pp. 137–161. - PubMed
-
- Stacey NJ, Roberts K, Carpita NC, Wells B, McCann MC. Dynamic changes in cell surface molecules are very early events in the differentiation of mesophyll cells from Zinnia elegans into tracheary elements. Plant J. 1995;8(6):891–906.
-
- Stacey NJ, Roberts K, Knox JP. Patterns of Expression of the Jim4 Arabinogalactan-Protein Epitope in Cell-Cultures and during Somatic Embryogenesis in Daucus-Carota L. Planta. 1990;180(2):285–292. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

