Epidermal growth factor receptor (EGFR) signaling is a key mediator of hormone-induced leukocyte infiltration in the pubertal female mammary gland
- PMID: 24693965
- PMCID: PMC4020926
- DOI: 10.1210/en.2013-1933
Epidermal growth factor receptor (EGFR) signaling is a key mediator of hormone-induced leukocyte infiltration in the pubertal female mammary gland
Abstract
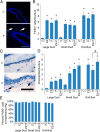
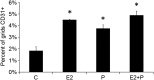
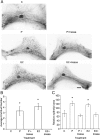
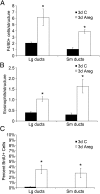
It is well documented that macrophages and eosinophils play important roles in normal murine pubertal mammary gland development. Although it is accepted that estrogen (E) and progesterone (P) are key players in mammary gland development, the roles these hormones might play in regulating the actions of leukocytes in that process is an understudied area. We show here that P and E, respectively, induce unique, but overlapping, sets of proinflammatory and angiogenic cytokines and chemokines, in the pubertal female BALB/c mammary gland, as well as induce infiltration of macrophages and eosinophils to the mammary periepithelium. This extends earlier studies showing P induction of proinflammatory products in pubertal and adult mammary epithelial organoids and P-induced in vivo infiltration of leukocytes to the adult mammary periepithelium. Importantly, epidermal growth factor receptor-signaling, which is likely mediated by amphiregulin (Areg), a downstream mediator of E and P, is both necessary and sufficient for both E- and P-induced recruitment of macrophages and eosinophils to the pubertal mammary periepithelium. We further show that receptor activator of nuclear factor κB ligand (RANKL), although not sufficient of itself to cause macrophage and eosinophil recruitment, contributes to an optimal response to P. The potency of Areg is highlighted by the fact that it is sufficient to induce macrophage and eosinophil recruitment at levels equivalent to that induced by either E or P. Our finding of a dominant role for Areg in hormonally induced leukocyte recruitment to the pubertal mammary gland parallels its dominance in regulating ductal outgrowth and its role in P-induced proliferation in the pubertal gland.
Figures

References
-
- Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127:2269–2282 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

