Adeno-associated virus serotypes 1, 8, and 9 share conserved mechanisms for anterograde and retrograde axonal transport
- PMID: 24694006
- PMCID: PMC4137353
- DOI: 10.1089/hum.2013.189
Adeno-associated virus serotypes 1, 8, and 9 share conserved mechanisms for anterograde and retrograde axonal transport
Abstract
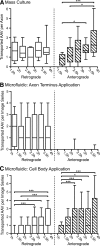
Adeno-associated virus (AAV) vectors often undergo long-distance axonal transport after brain injection. This leads to transduction of brain regions distal to the injection site, although the extent of axonal transport and distal transduction varies widely among AAV serotypes. The mechanisms driving this variability are poorly understood. This is a critical problem for applications that require focal gene expression within a specific brain region, and also impedes the utilization of vector transport for applications requiring widespread delivery of transgene to the brain. Here, we compared AAV serotypes 1 and 9, which frequently demonstrate distal transduction, with serotype 8, which rarely spreads beyond the injection site. To examine directional AAV transport in vitro, we used a microfluidic chamber to apply dye-labeled AAV to the axon termini or to the cell bodies of primary rat embryonic cortical neurons. All three serotypes were actively transported along axons, with transport characterized by high velocities and prolonged runs in both the anterograde and retrograde directions. Coinfection with pairs of serotypes indicated that AAV1, 8, and 9 share the same intracellular compartments for axonal transport. In vivo, both AAV8 and 9 demonstrated anterograde and retrograde transport within a nonreciprocal circuit after injection into adult mouse brain, with highly similar distributions of distal transduction. However, in mass-cultured neurons, we found that AAV1 was more frequently transported than AAV8 or 9, and that the frequency of AAV9 transport could be enhanced by increasing receptor availability. Thus, while these serotypes share conserved mechanisms for axonal transport both in vitro and in vivo, the frequency of transport can vary among serotypes, and axonal transport can be markedly increased by enhancing vector uptake. This suggests that variability in distal transduction in vivo likely results from differential uptake at the plasma membrane, rather than fundamental differences in transport mechanisms among AAV serotypes.
Figures






References
-
- Atchison R.W., Casto B.C., and Hammon W.M. (1965). Adenovirus-associated defective virus particles. Science 149, 754–756 - PubMed
-
- Broekman M.L., Comer L.A., Hyman B.T., and Sena-Esteves M. (2006). Adeno-associated virus vectors serotyped with AAV8 capsid are more efficient than AAV-1 or -2 serotypes for widespread gene delivery to the neonatal mouse brain. Neuroscience 138, 501–510 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

