The Ca2+-activated Cl- channel ANO1/TMEM16A regulates primary ciliogenesis
- PMID: 24694595
- PMCID: PMC4038505
- DOI: 10.1091/mbc.E13-10-0599
The Ca2+-activated Cl- channel ANO1/TMEM16A regulates primary ciliogenesis
Abstract
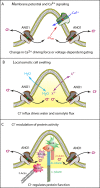
Many cells possess a single, nonmotile, primary cilium highly enriched in receptors and sensory transduction machinery that plays crucial roles in cellular morphogenesis. Although sensory transduction requires ion channels, relatively little is known about ion channels in the primary cilium (with the exception of TRPP2). Here we show that the Ca(2+)-activated Cl ((-)) channel anoctamin-1 (ANO1/TMEM16A) is located in the primary cilium and that blocking its channel function pharmacologically or knocking it down with short hairpin RNA interferes with ciliogenesis. Before ciliogenesis, the channel becomes organized into a torus-shaped structure ("the nimbus") enriched in proteins required for ciliogenesis, including the small GTPases Cdc42 and Arl13b and the exocyst complex component Sec6. The nimbus excludes F-actin and coincides with a ring of acetylated microtubules. The nimbus appears to form before, or independent of, apical docking of the mother centriole. Our data support a model in which the nimbus provides a scaffold for staging of ciliary components for assembly very early in ciliogenesis and chloride transport by ANO1/TMEM16A is required for the genesis or maintenance of primary cilia.
© 2014 Ruppersburg and Hartzell. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures






Similar articles
-
Anoctamin 1 (Tmem16A) Ca2+-activated chloride channel stoichiometrically interacts with an ezrin-radixin-moesin network.Proc Natl Acad Sci U S A. 2012 Jun 26;109(26):10376-81. doi: 10.1073/pnas.1200174109. Epub 2012 Jun 8. Proc Natl Acad Sci U S A. 2012. PMID: 22685202 Free PMC article.
-
Expression and function of Anoctamin 1/TMEM16A calcium-activated chloride channels in airways of in vivo mouse models for cystic fibrosis research.Pflugers Arch. 2018 Sep;470(9):1335-1348. doi: 10.1007/s00424-018-2160-x. Epub 2018 Jun 2. Pflugers Arch. 2018. PMID: 29860639
-
Relationship between TMEM16A/anoctamin 1 and LRRC8A.Pflugers Arch. 2016 Oct;468(10):1751-63. doi: 10.1007/s00424-016-1862-1. Epub 2016 Aug 12. Pflugers Arch. 2016. PMID: 27514381
-
Bestrophin and TMEM16-Ca(2+) activated Cl(-) channels with different functions.Cell Calcium. 2009 Oct;46(4):233-41. doi: 10.1016/j.ceca.2009.09.003. Epub 2009 Sep 26. Cell Calcium. 2009. PMID: 19783045 Review.
-
The Ca(2+) -activated Cl(-) channel, ANO1 (TMEM16A), is a double-edged sword in cell proliferation and tumorigenesis.Cancer Med. 2014 Jun;3(3):453-61. doi: 10.1002/cam4.232. Epub 2014 Mar 18. Cancer Med. 2014. PMID: 24639373 Free PMC article. Review.
Cited by
-
Identification of a lipid scrambling domain in ANO6/TMEM16F.Elife. 2015 Jun 9;4:e06901. doi: 10.7554/eLife.06901. Elife. 2015. PMID: 26057829 Free PMC article.
-
Primary cilia proteins: ciliary and extraciliary sites and functions.Cell Mol Life Sci. 2018 May;75(9):1521-1540. doi: 10.1007/s00018-017-2740-5. Epub 2018 Jan 5. Cell Mol Life Sci. 2018. PMID: 29305615 Free PMC article. Review.
-
TMEM16A drives renal cyst growth by augmenting Ca2+ signaling in M1 cells.J Mol Med (Berl). 2020 May;98(5):659-671. doi: 10.1007/s00109-020-01894-y. Epub 2020 Mar 18. J Mol Med (Berl). 2020. PMID: 32185407 Free PMC article.
-
Primary Cilia-An Underexplored Topic in Major Mental Illness.Front Psychiatry. 2019 Mar 4;10:104. doi: 10.3389/fpsyt.2019.00104. eCollection 2019. Front Psychiatry. 2019. PMID: 30886591 Free PMC article. Review.
-
Progress in ciliary ion channel physiology.J Gen Physiol. 2017 Jan;149(1):37-47. doi: 10.1085/jgp.201611696. Epub 2016 Dec 20. J Gen Physiol. 2017. PMID: 27999145 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

