Primary cilia in stem cells and neural progenitors are regulated by neutral sphingomyelinase 2 and ceramide
- PMID: 24694597
- PMCID: PMC4038499
- DOI: 10.1091/mbc.E13-12-0730
Primary cilia in stem cells and neural progenitors are regulated by neutral sphingomyelinase 2 and ceramide
Abstract
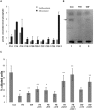
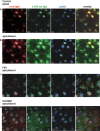
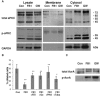
We show here that human embryonic stem (ES) and induced pluripotent stem cell-derived neuroprogenitors (NPs) develop primary cilia. Ciliogenesis depends on the sphingolipid ceramide and its interaction with atypical PKC (aPKC), both of which distribute to the primary cilium and the apicolateral cell membrane in NP rosettes. Neural differentiation of human ES cells to NPs is concurrent with a threefold elevation of ceramide-in particular, saturated, long-chain C16:0 ceramide (N-palmitoyl sphingosine) and nonsaturated, very long chain C24:1 ceramide (N-nervonoyl sphingosine). Decreasing ceramide levels by inhibiting ceramide synthase or neutral sphingomyelinase 2 leads to translocation of membrane-bound aPKC to the cytosol, concurrent with its activation and the phosphorylation of its substrate Aurora kinase A (AurA). Inhibition of aPKC, AurA, or a downstream target of AurA, HDAC6, restores ciliogenesis in ceramide-depleted cells. Of importance, addition of exogenous C24:1 ceramide reestablishes membrane association of aPKC, restores primary cilia, and accelerates neural process formation. Taken together, these results suggest that ceramide prevents activation of HDAC6 by cytosolic aPKC and AurA, which promotes acetylation of tubulin in primary cilia and, potentially, neural processes. This is the first report on the critical role of ceramide generated by nSMase2 in stem cell ciliogenesis and differentiation.
© 2014 He et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures




References
-
- Aubin I, et al. A deletion in the gene encoding sphingomyelin phosphodiesterase 3 (Smpd3) results in osteogenesis and dentinogenesis imperfecta in the mouse. Nat Genet. 2005;37:803–805. - PubMed
-
- Bieberich E, Kawaguchi T, Yu RK. N-acylated serinol is a novel ceramide mimic inducing apoptosis in neuroblastoma cells. J Biol Chem. 2000;275:177–181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

