Protein tyrosine phosphatases ε and α perform nonredundant roles in osteoclasts
- PMID: 24694598
- PMCID: PMC4038506
- DOI: 10.1091/mbc.E14-03-0788
Protein tyrosine phosphatases ε and α perform nonredundant roles in osteoclasts
Abstract
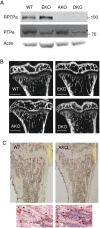
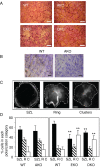
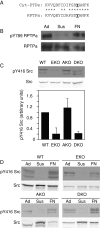
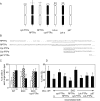
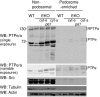
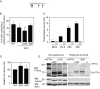
Female mice lacking protein tyrosine phosphatase ε (PTP ε) are mildly osteopetrotic. Osteoclasts from these mice resorb bone matrix poorly, and the structure, stability, and cellular organization of their podosomal adhesion structures are abnormal. Here we compare the role of PTP ε with that of the closely related PTP α in osteoclasts. We show that bone mass and bone production and resorption, as well as production, structure, function, and podosome organization of osteoclasts, are unchanged in mice lacking PTP α. The varying effects of either PTP on podosome organization in osteoclasts are caused by their distinct N-termini. Osteoclasts express the receptor-type PTP α (RPTPa), which is absent from podosomes, and the nonreceptor form of PTP ε (cyt-PTPe), which is present in these structures. The presence of the unique 12 N-terminal residues of cyt-PTPe is essential for podosome regulation; attaching this sequence to the catalytic domains of PTP α enables them to function in osteoclasts. Serine 2 within this sequence regulates cyt-PTPe activity and its effects on podosomes. We conclude that PTPs α and ε play distinct roles in osteoclasts and that the N-terminus of cyt-PTPe, in particular serine 2, is critical for its function in these cells.
© 2014 Finkelshtein et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures
References
-
- Amoui M, Sheng MH, Chen ST, Baylink DJ, Lau KH. A transmembrane osteoclastic protein-tyrosine phosphatase regulates osteoclast activity in part by promoting osteoclast survival through c-Src-dependent activation of NFkappaB and JNK2. Arch Biochem Biophys. 2007;463:47–59. - PubMed
-
- Aoki K, Didomenico E, Sims NA, Mukhopadhyay K, Neff L, Houghton A, Amling M, Levy JB, Horne WC, Baron R. The tyrosine phosphatase SHP-1 is a negative regulator of osteoclastogenesis and osteoclast resorbing activity: increased resorption and osteopenia in me(v)/me(v) mutant mice. Bone. 1999;25:261–267. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

