Virologic and serologic outcomes of mono versus dual HBV therapy and characterization of HIV/HBV coinfection in a US cohort
- PMID: 24694927
- PMCID: PMC4169110
- DOI: 10.1097/QAI.0000000000000149
Virologic and serologic outcomes of mono versus dual HBV therapy and characterization of HIV/HBV coinfection in a US cohort
Abstract
Objectives: To characterize HIV/hepatitis B virus (HBV) coinfection in the AIDS Clinical Trials Group Longitudinal Linked Randomized Trials cohort and compare long-term HBV outcomes between regimens with 1 (MONO) or 2 (DUAL) anti-HBV agents.
Design: A retrospective study of coinfected AIDS Clinical Trials Group Longitudinal Linked Randomized Trials subjects who received regimens containing anti-HBV agent(s).
Methods: Stored samples at baseline and weeks 16, 32, 48, 144, and 240 were tested for HBV DNA, HBV e antigen (HBeAg), HBV e antibody (HBeAb), and hepatitis D virus (HDV) antibody. Resistance and genotype were tested in samples with HBV DNA >600 IU/mL. MONO versus DUAL analyses were limited to HBV treatment-naive subjects (Naive-MONO, Naive-DUAL).
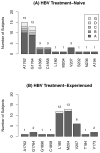
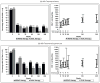
Results: Of 150 study subjects, median age was 40 years, 96% were male; 57% white, 26% black, 13% Hispanic. Baseline median CD4 was 224 cells per cubic millimeter, HIV RNA 4.48 log10 copies/mL, HBV DNA 6.30 log10 IU/mL; 59% HBeAg positive and 65% HBeAb negative; HBV genotypes A = 69%, G = 18%, D = 7%, <2% for A/G, B, C, F, H. Coinfection with HDV was 2%. There were 49 Naive-MONO (lamivudine) and 22 Naive-DUAL (11 lamivudine + tenofovir, 11 emtricitabine + tenofovir) with detectable HBV DNA. In the 240-week follow-up, HBV DNA suppression was not significantly higher in Naive-DUAL (P = 0.14); lower baseline HBV DNA (P < 0.01) was associated with suppression. Among 32 Naive-MONO subjects with detectable HBV DNA at baseline and results at week 48, 41% suppressed; among such 15 Naive-DUAL subjects, 53% suppressed. HBeAg and HBeAb analyses showed similar trends.
Conclusions: While consistent trends toward increased HBV DNA suppression, HBeAg loss and HBeAb seroconversion were observed in Naive-DUAL compared with Naive-MONO, they were not statistically significant. Overall, HDV coinfection was low.
Conflict of interest statement
Conflicts: Dr. Aberg serves on AbbVie, Janssen and Merck scientific advisory boards; Dr. Aberg has moved affiliation to the ICAHN School of Medicine at Mount Sinai.
Figures

Similar articles
-
Five-year on-treatment efficacy of lamivudine-, tenofovir- and tenofovir + emtricitabine-based HAART in HBV-HIV-coinfected patients.J Viral Hepat. 2012 Nov;19(11):801-10. doi: 10.1111/j.1365-2893.2012.01601.x. Epub 2012 Mar 15. J Viral Hepat. 2012. PMID: 23043387
-
Patterns and causes of suboptimal response to tenofovir-based therapy in individuals coinfected with HIV and hepatitis B virus.Clin Infect Dis. 2013 May;56(9):e87-94. doi: 10.1093/cid/cit002. Epub 2013 Jan 11. Clin Infect Dis. 2013. PMID: 23315316 Free PMC article.
-
Lamivudine Monotherapy-Based cART Is Efficacious for HBV Treatment in HIV/HBV Coinfection When Baseline HBV DNA <20,000 IU/mL.J Acquir Immune Defic Syndr. 2016 May 1;72(1):39-45. doi: 10.1097/QAI.0000000000000927. J Acquir Immune Defic Syndr. 2016. PMID: 26745828 Free PMC article.
-
[Hepatitis B in patients with HIV infection].Enferm Infecc Microbiol Clin. 2008 May;26 Suppl 7:71-9. doi: 10.1016/s0213-005x(08)76522-4. Enferm Infecc Microbiol Clin. 2008. PMID: 19100234 Review. Spanish.
-
Antiretroviral therapy for hepatitis B virus-HIV-coinfected patients: promises and pitfalls.Clin Infect Dis. 2006 Oct 1;43(7):904-10. doi: 10.1086/507532. Epub 2006 Aug 23. Clin Infect Dis. 2006. PMID: 16941375 Review.
Cited by
-
Prevalence of hepatitis B and C viruses in HIV-positive patients in China: a cross-sectional study.J Int AIDS Soc. 2016 Mar 14;19(1):20659. doi: 10.7448/IAS.19.1.20659. eCollection 2016. J Int AIDS Soc. 2016. PMID: 26979535 Free PMC article.
-
Comparative effectiveness of anti-viral drugs with dual activity for treating hepatitis B and HIV co-infected patients: a network meta-analysis.BMC Infect Dis. 2018 Nov 14;18(1):564. doi: 10.1186/s12879-018-3506-x. BMC Infect Dis. 2018. PMID: 30428847 Free PMC article.
-
Hepatitis B and HIV co-infection is still treated using lamivudine-only antiretroviral therapy combination in Uganda.Afr Health Sci. 2015 Jun;15(2):328-33. doi: 10.4314/ahs.v15i2.4. Afr Health Sci. 2015. PMID: 26124776 Free PMC article.
-
Coinfection Dynamics of Two Diseases in a Single Host Population.J Math Anal Appl. 2016 Oct 1;442(1):171-188. doi: 10.1016/j.jmaa.2016.04.039. Epub 2016 Apr 19. J Math Anal Appl. 2016. PMID: 27667856 Free PMC article.
-
Long-term virological and serologic responses of chronic hepatitis B virus infection to tenofovir disoproxil fumarate-containing regimens in patients with HIV and hepatitis B coinfection.Hepatol Int. 2019 Jul;13(4):431-439. doi: 10.1007/s12072-019-09953-4. Epub 2019 Jun 8. Hepatol Int. 2019. PMID: 31177505
References
-
- Soriano V, Barreiro P, Sherman KE. The Changing Epidemiology of Liver Disease in HIV Patients. AIDS reviews. 2013 Jan-Mar;15(1):25–31. - PubMed
-
- Puoti M, Moioli MC, Travi G, Rossotti R. The burden of liver disease in human immunodeficiency virus-infected patients. Seminars in liver disease. 2012 May;32(2):103–113. - PubMed
-
- Iser DM, Lewin SR. The pathogenesis of liver disease in the setting of HIV-hepatitis B virus coinfection. Antiviral therapy. 2009;14(2):155–164. - PubMed
-
- Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009 Apr 10;58(RR-4):1–207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UM1 AI069494/AI/NIAID NIH HHS/United States
- UM1AI068634/AI/NIAID NIH HHS/United States
- AI069532/AI/NIAID NIH HHS/United States
- U01 AI069532/AI/NIAID NIH HHS/United States
- 5R21AI083106/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
- UM1 AI069532/AI/NIAID NIH HHS/United States
- UM1AI068636/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- R21 AI083106/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

