A mesoscale connectome of the mouse brain
- PMID: 24695228
- PMCID: PMC5102064
- DOI: 10.1038/nature13186
A mesoscale connectome of the mouse brain
Abstract
Comprehensive knowledge of the brain's wiring diagram is fundamental for understanding how the nervous system processes information at both local and global scales. However, with the singular exception of the C. elegans microscale connectome, there are no complete connectivity data sets in other species. Here we report a brain-wide, cellular-level, mesoscale connectome for the mouse. The Allen Mouse Brain Connectivity Atlas uses enhanced green fluorescent protein (EGFP)-expressing adeno-associated viral vectors to trace axonal projections from defined regions and cell types, and high-throughput serial two-photon tomography to image the EGFP-labelled axons throughout the brain. This systematic and standardized approach allows spatial registration of individual experiments into a common three dimensional (3D) reference space, resulting in a whole-brain connectivity matrix. A computational model yields insights into connectional strength distribution, symmetry and other network properties. Virtual tractography illustrates 3D topography among interconnected regions. Cortico-thalamic pathway analysis demonstrates segregation and integration of parallel pathways. The Allen Mouse Brain Connectivity Atlas is a freely available, foundational resource for structural and functional investigations into the neural circuits that support behavioural and cognitive processes in health and disease.
Conflict of interest statement
The authors declare no competing financial interests. Readers are welcome to comment on the online version of the paper.
Figures
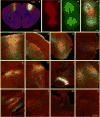




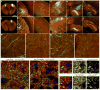
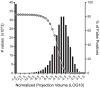

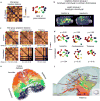







Comment in
-
Major progress towards elucidating brain wiring diagrams.J Comp Neurol. 2014 Jun 15;522(9):1987-8. doi: 10.1002/cne.23586. J Comp Neurol. 2014. PMID: 24752413 No abstract available.
-
From connections to function: the mouse brain connectome atlas.Cell. 2014 May 8;157(4):773-5. doi: 10.1016/j.cell.2014.04.023. Cell. 2014. PMID: 24813604
-
Chasing the dreams of early connectionists.ACS Chem Neurosci. 2014 Jul 16;5(7):491-3. doi: 10.1021/cn5000937. Epub 2014 May 9. ACS Chem Neurosci. 2014. PMID: 24814363 Free PMC article.
References
-
- Swanson LW. Brain Architecture: Understanding the Basic Plan. Vol. 331. Oxford Univ. Press; 2012.
-
- Seung S. Connectome: How The Brain’s Wiring Makes Us Who We Are. Vol. 359. Houghton Mifflin Harcourt; 2012.
-
- Sporns O. Networks of the Brain. Vol. 412. The MIT Press; 2011.
-
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Phil Trans R Soc Lond B. 1986;314:1–340. - PubMed
-
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

