Transcriptional landscape of the prenatal human brain
- PMID: 24695229
- PMCID: PMC4105188
- DOI: 10.1038/nature13185
Transcriptional landscape of the prenatal human brain
Abstract
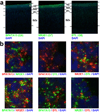
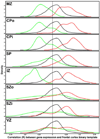
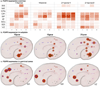
The anatomical and functional architecture of the human brain is mainly determined by prenatal transcriptional processes. We describe an anatomically comprehensive atlas of the mid-gestational human brain, including de novo reference atlases, in situ hybridization, ultra-high-resolution magnetic resonance imaging (MRI) and microarray analysis on highly discrete laser-microdissected brain regions. In developing cerebral cortex, transcriptional differences are found between different proliferative and post-mitotic layers, wherein laminar signatures reflect cellular composition and developmental processes. Cytoarchitectural differences between human and mouse have molecular correlates, including species differences in gene expression in subplate, although surprisingly we find minimal differences between the inner and outer subventricular zones even though the outer zone is expanded in humans. Both germinal and post-mitotic cortical layers exhibit fronto-temporal gradients, with particular enrichment in the frontal lobe. Finally, many neurodevelopmental disorder and human-evolution-related genes show patterned expression, potentially underlying unique features of human cortical formation. These data provide a rich, freely-accessible resource for understanding human brain development.
Figures





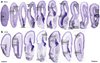






References
References (for online methods)
-
- Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics (Oxford, England) 2007;8:118–127. - PubMed
-
- Huang DW, Sherman B, Lempicki R. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protocols. 2009;4:44–57. - PubMed

