Autologous cord blood therapy for infantile cerebral palsy: from bench to bedside
- PMID: 24695413
- PMCID: PMC3956288
- DOI: 10.1155/2014/976321
Autologous cord blood therapy for infantile cerebral palsy: from bench to bedside
Abstract
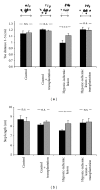
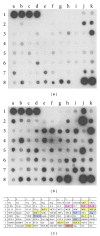
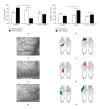
About 17 million people worldwide live with cerebral palsy, the most common disability in childhood, with hypoxic-ischemic encephalopathy, preterm birth, and low birth weight being the most important risk factors. This review will focus on recent developments in cell therapy for infantile cerebral palsy by transplantation of autologous umbilical cord blood. There are only 4 publications available at present; however, the observations made along with experimental data in vivo and in vitro may be of utmost importance clinically, so that a review at an early developmental stage of this new therapeutic concept seems justified. Particularly, since the first published double-blind randomized placebo-controlled trial in a paradigm using allogeneic cord blood and erythropoietin to treat cerebral palsy under immunosuppression showed beneficial therapeutic effects in infantile cerebral palsy, long-held doubts about the efficacy of this new cell therapy are dispelled and a revision of therapeutic views upon an ailment, for which there is no cure at present, is warranted. Hence, this review will summarize the available information on autologous cord blood therapy for cerebral palsy and that on the relevant experimental work as far as potential mechanisms and modes of action are concerned.
Figures






References
-
- Kancherla V, Amendah DD, Grosse SD, Yeargin-Allsopp M, van Naarden Braun K. Medical expenditures attributable to cerebral palsy and intellectual disability among Medicaid-enrolled children. Research in Developmental Disabilities. 2012;33(3):832–840. - PubMed
-
- Novak I, Hines M, Goldsmith S, Barclay R. Clinical prognostic messages from a systematic review on cerebral palsy. Pediatrics. 2012;130(5):e1285–e1312. - PubMed
-
- Struck A, Almaazmi M, Bode H, et al. Neurodevelopmental outcome of very low birth weight infants born at the Perinatal Centre in Ulm, Germany. Zeitschrift für Geburtshilfe und Neonatologie. 2013;217(2):65–71. - PubMed
-
- Irazábal M, Marsà F, García M, et al. Family burden related to clinical and functional variables of people with intellectual disability with and without a mental disorder. Research in Developmental Disabilities. 2012;33(3):796–803. - PubMed
-
- Centers for Disease Control and Prevention (CDC) Economic costs associated with mental retardation, cerebral palsy, hearing loss, and vision impairment—United States. Morbidity and Mortality Weekly Report. 2003;53:57–59. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

