Epidermal or dermal specific knockout of PHD-2 enhances wound healing and minimizes ischemic injury
- PMID: 24695462
- PMCID: PMC3973687
- DOI: 10.1371/journal.pone.0093373
Epidermal or dermal specific knockout of PHD-2 enhances wound healing and minimizes ischemic injury
Abstract
Introduction: Hypoxia-inducible factor (HIF)-1α, part of the heterodimeric transcription factor that mediates the cellular response to hypoxia, is critical for the expression of multiple angiogenic growth factors, cell motility, and the recruitment of endothelial progenitor cells. Inhibition of the oxygen-dependent negative regulator of HIF-1α, prolyl hydroxylase domain-2 (PHD-2), leads to increased HIF-1α and mimics various cellular and physiological responses to hypoxia. The roles of PHD-2 in the epidermis and dermis have not been clearly defined in wound healing.
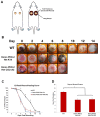
Methods: Epidermal and dermal specific PHD-2 knockout (KO) mice were developed in a C57BL/6J (wild type) background by crossing homozygous floxed PHD-2 mice with heterozygous K14-Cre mice and heterozygous Col1A2-Cre-ER mice to get homozygous floxed PHD-2/heterozygous K14-Cre and homozygous floxed PHD-2/heterozygous floxed Col1A2-Cre-ER mice, respectively. Ten to twelve-week-old PHD-2 KO and wild type (WT) mice were subjected to wounding and ischemic pedicle flap model. The amount of healing was grossly quantified with ImageJ software. Western blot and qRT-PCR was run on protein and RNA from primary cells cultured in vitro.
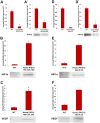
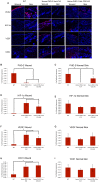
Results: qRT-PCR demonstrated a significant decrease of PHD-2 in keratinocytes and fibroblasts derived from tissue specific KO mice relative to control mice (*p<0.05). Western blot analysis showed a significant increase in HIF-1α and VEGF protein levels in PHD-2 KO mice relative to control mice (*p<0.05). PHD-2 KO mice showed significantly accelerated wound closure relative to WT (*p<0.05). When ischemia was analyzed at day nine post-surgery in a flap model, the PHD-2 tissue specific knockout mice showed significantly more viable flaps than WT (*p<0.05).
Conclusions: PHD-2 plays a significant role in the rates of wound healing and response to ischemic insult in mice. Further exploration shows PHD-2 KO increases cellular levels of HIF-1α and this increase leads to the transcription of downstream angiogenic factors such as VEGF.
Conflict of interest statement
Figures

References
-
- Kranke P, Bennett MH, Martyn-St James M, Schnabel A, Debus SE (2012) Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev 4: CD004123. - PubMed
-
- Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, et al. (2008) Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol 58: 185–206. - PubMed
-
- Kirsner RS, Warriner R, Michela M, Stasik L, Freeman K (2010) Advanced biological therapies for diabetic foot ulcers. Arch Dermatol 146: 857–862. - PubMed
-
- Driver VR, Fabbi M, Lavery LA, Gibbons G (2010) The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg 52: 17S–22S. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

