Identification of odorant-receptor interactions by global mapping of the human odorome
- PMID: 24695519
- PMCID: PMC3973694
- DOI: 10.1371/journal.pone.0093037
Identification of odorant-receptor interactions by global mapping of the human odorome
Abstract
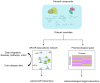
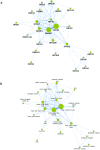
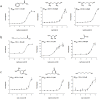
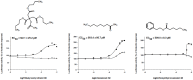
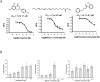
The human olfactory system recognizes a broad spectrum of odorants using approximately 400 different olfactory receptors (hORs). Although significant improvements of heterologous expression systems used to study interactions between ORs and odorant molecules have been made, screening the olfactory repertoire of hORs remains a tremendous challenge. We therefore developed a chemical systems level approach based on protein-protein association network to investigate novel hOR-odorant relationships. Using this new approach, we proposed and validated new bioactivities for odorant molecules and OR2W1, OR51E1 and OR5P3. As it remains largely unknown how human perception of odorants influence or prevent diseases, we also developed an odorant-protein matrix to explore global relationships between chemicals, biological targets and disease susceptibilities. We successfully experimentally demonstrated interactions between odorants and the cannabinoid receptor 1 (CB1) and the peroxisome proliferator-activated receptor gamma (PPARγ). Overall, these results illustrate the potential of integrative systems chemical biology to explore the impact of odorant molecules on human health, i.e. human odorome.
Conflict of interest statement
Figures
References
-
- Buck L, Axel R (1991) A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell 65: 175–187. - PubMed
-
- Matsui A, Go Y, Niimura Y (2010) Degeneration of olfactory receptor gene repertories in primates: No direct link to full trichromatic vision. Mol Biol Evol 27: 1192–1200. - PubMed
-
- Kraft P, Bajgrowicz JA, Denis C, Frater G (2000) Odds and trends: Recent developments in the chemistry of odorants. Angew Chem-Int Edit 39: 2981–3010. - PubMed
-
- Zarzo M (2007) The sense of smell: Molecular basis of odorant recognition. Biol Rev 82: 455–479. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

