IL-18 attenuates experimental choroidal neovascularization as a potential therapy for wet age-related macular degeneration
- PMID: 24695684
- PMCID: PMC12016488
- DOI: 10.1126/scitranslmed.3007616
IL-18 attenuates experimental choroidal neovascularization as a potential therapy for wet age-related macular degeneration
Abstract
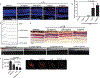
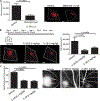
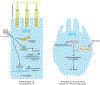
Age-related macular degeneration (AMD) is the most common form of central retinal blindness globally. Distinct processes of the innate immune system, specifically activation of the NLRP3 inflammasome, have been shown to play a central role in the development of both "dry" and neovascular ("wet") forms of the disease. We show that the inflammatory cytokine interleukin-18 (IL-18) can regulate choroidal neovascularization formation in mice. We observed that exogenous administration of mature recombinant IL-18 has no effect on retinal pigment epithelial (RPE) cell viability, but that overexpression of pro-IL-18 or pro-IL-1β alone can cause RPE cell swelling and subsequent atrophy, a process that can be inhibited by the promotion of autophagy. A direct comparison of local and systemic administration of mature recombinant IL-18 with current anti-VEGF (vascular endothelial growth factor)-based therapeutic strategies shows that IL-18 treatment works effectively alone and more effectively in combination with anti-VEGF therapy and represents a novel therapeutic strategy for the treatment of wet AMD.
Conflict of interest statement
Figures




Comment in
-
Eye diseases: a new use for interleukin-18 in sight.Nat Rev Drug Discov. 2014 Jun;13(6):418. doi: 10.1038/nrd4342. Epub 2014 May 16. Nat Rev Drug Discov. 2014. PMID: 24833293 No abstract available.
-
IL-18 is not therapeutic for neovascular age-related macular degeneration.Nat Med. 2014 Dec;20(12):1372-5. doi: 10.1038/nm.3671. Nat Med. 2014. PMID: 25473914 Free PMC article. No abstract available.
-
Reply to IL-18 is not therapeutic for neovascular age-related macular degeneration.Nat Med. 2014 Dec;20(12):1376-7. doi: 10.1038/nm.3741. Nat Med. 2014. PMID: 25473915 No abstract available.
References
-
- Rein DB, Wittenborn JS, Zhang X, Honeycutt AA, Lesesne SB, Saaddine J; Vision Health Cost-Effectiveness Study Group, Forecasting age-related macular degeneration through the year 2050: The potential impact of new treatments. Arch. Ophthalmol. 127, 533–540 (2009). - PubMed
-
- Janeway CA, Medzhitov R, Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002). - PubMed
-
- O’Neill LA, Bowie AG, The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 7, 353–364 (2007). - PubMed
-
- Schroder K, Tschopp J, The inflammasomes. Cell 140, 821–832 (2010). - PubMed
-
- Jager RD, Mieler WF, Miller JW, Age-related macular degeneration. N. Engl. J. Med. 358, 2606–2617 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

