14-3-3 proteins are required for hippocampal long-term potentiation and associative learning and memory
- PMID: 24695700
- PMCID: PMC3972712
- DOI: 10.1523/JNEUROSCI.4393-13.2014
14-3-3 proteins are required for hippocampal long-term potentiation and associative learning and memory
Abstract
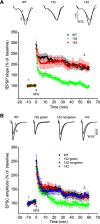
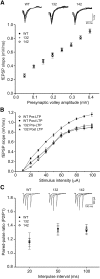
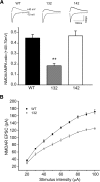
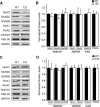
14-3-3 is a family of regulatory proteins highly expressed in the brain. Previous invertebrate studies have demonstrated the importance of 14-3-3 in the regulation of synaptic functions and learning and memory. However, the in vivo role of 14-3-3 in these processes has not been determined using mammalian animal models. Here, we report the behavioral and electrophysiological characterization of a new animal model of 14-3-3 proteins. These transgenic mice, considered to be a 14-3-3 functional knock-out, express a known 14-3-3 inhibitor in various brain regions of different founder lines. We identify a founder-specific impairment in hippocampal-dependent learning and memory tasks, as well as a correlated suppression in long-term synaptic plasticity of the hippocampal synapses. Moreover, hippocampal synaptic NMDA receptor levels are selectively reduced in the transgenic founder line that exhibits both behavioral and synaptic plasticity deficits. Collectively, our findings provide evidence that 14-3-3 is a positive regulator of associative learning and memory at both the behavioral and cellular level.
Keywords: 14-3-3 proteins; NMDA receptors; fear conditioning; long-term potentiation; passive avoidance.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
