Protein splicing: how inteins escape from precursor proteins
- PMID: 24695729
- PMCID: PMC4031507
- DOI: 10.1074/jbc.R113.540310
Protein splicing: how inteins escape from precursor proteins
Abstract
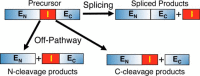
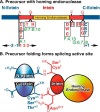
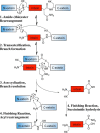
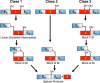
Inteins are nature's escape artists; they facilitate their excision from flanking polypeptides (exteins) concomitant with extein ligation to produce a mature host protein. Splicing requires sequential nucleophilic displacement reactions catalyzed by strategies similar to proteases and asparagine lyases. Inteins require precise reaction coordination rather than rapid turnover or tight substrate binding because they are single turnover enzymes with covalently linked substrates. This has allowed inteins to explore alternative mechanisms with different steps or to use different methods for activation and coordination of the steps. Pressing issues include understanding the underlying details of catalysis and how the splicing steps are controlled.
Keywords: Asparagine Cyclization; Bacterial Intein-like Domain; Enzyme Kinetics; Enzyme Mechanisms; Hedgehog; Intein; Post-translational Modification; Protein Motifs; Protein Splicing; Thioester.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
References
-
- Hirata R., Ohsumk Y., Nakano A., Kawasaki H., Suzuki K., Anraku Y. (1990) Molecular structure of a gene, VMA1, encoding the catalytic subunit of H+-translocating adenosine triphosphatase from vacuolar membranes of Saccharomyces cerevisiae. J. Biol. Chem. 265, 6726–6733 - PubMed
-
- Kane P. M., Yamashiro C. T., Wolczyk D. F., Neff N., Goebl M., Stevens T. H. (1990) Protein splicing converts the yeast TFP1 gene product to the 69-kD subunit of the vacuolar H+-adenosine triphosphatase. Science 250, 651–657 - PubMed
-
- Davis E. O., Jenner P. J., Brooks P. C., Colston M. J., Sedgwick S. G. (1992) Protein splicing in the maturation of M. tuberculosis recA protein: a mechanism for tolerating a novel class of intervening sequence. Cell 71, 201–210 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

