ELIC-α7 Nicotinic acetylcholine receptor (α7nAChR) chimeras reveal a prominent role of the extracellular-transmembrane domain interface in allosteric modulation
- PMID: 24695730
- PMCID: PMC4022858
- DOI: 10.1074/jbc.M113.524611
ELIC-α7 Nicotinic acetylcholine receptor (α7nAChR) chimeras reveal a prominent role of the extracellular-transmembrane domain interface in allosteric modulation
Abstract
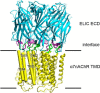
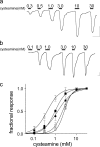
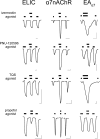
The native α7 nicotinic acetylcholine receptor (α7nAChR) is a homopentameric ligand-gated ion channel mediating fast synaptic transmission and is of pharmaceutical interest for treatment of numerous disorders. The transmembrane domain (TMD) of α7nAChR has been identified as a target for positive allosteric modulators (PAMs), but it is unclear whether modulation occurs through changes entirely within the TMD or changes involving both the TMD and the extracellular domain (ECD)-TMD interface. In this study, we constructed multiple chimeras using the TMD of human α7nAChR and the ECD of a prokaryotic homolog, ELIC, which is not sensitive to these modulators, and for which a high resolution structure has been solved. Functional ELIC-α7nAChR (EA) chimeras were obtained when their ECD-TMD interfaces were modified to resemble either the ELIC interface (EAELIC) or α7nAChR interface (EAα7). Both EAα7 and EAELIC show similar activation response and desensitization characteristics, but only EAα7 retained the unique pharmacology of α7nAChR evoked by PAMs, including potentiation by ivermectin, PNU-120596, and TQS, as well as activation by 4BP-TQS. This study suggests that PAM modulation through the TMD has a more stringent requirement at the ECD-TMD interface than agonist activation.
Keywords: Allosteric Regulation; ELIC; Ion Channels; Neurotransmitter Receptors; Nicotinic Acetylcholine Receptors; Pentameric Ligand-gated Ion Channels; Protein Chimeras; pLGICs; α7nAChR.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Couturier S., Bertrand D., Matter J. M., Hernandez M. C., Bertrand S., Millar N., Valera S., Barkas T., Ballivet M. (1990) A neuronal nicotinic acetylcholine receptor subunit (α7) is developmentally regulated and forms a homo-oligomeric channel blocked by α-BTX. Neuron 5, 847–856 - PubMed
-
- Alsharari S. D., Freitas K., Damaj M. I. (2013) Functional role of α7 nicotinic receptor in chronic neuropathic and inflammatory pain: studies in transgenic mice. Biochem. Pharmacol. 86, 1201–1207 - PubMed
-
- Wang H., Yu M., Ochani M., Amella C. A., Tanovic M., Susarla S., Li J. H., Wang H., Yang H., Ulloa L., Al-Abed Y., Czura C. J., Tracey K. J. (2003) Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 421, 384–388 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

