The Parkinson disease-linked LRRK2 protein mutation I2020T stabilizes an active state conformation leading to increased kinase activity
- PMID: 24695735
- PMCID: PMC4036318
- DOI: 10.1074/jbc.M113.537811
The Parkinson disease-linked LRRK2 protein mutation I2020T stabilizes an active state conformation leading to increased kinase activity
Abstract
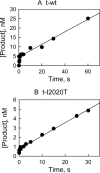
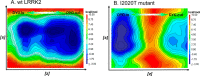
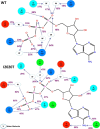
The effect of leucine-rich repeat kinase 2 (LRRK2) mutation I2020T on its kinase activity has been controversial, with both increased and decreased effects being reported. We conducted steady-state and pre-steady-state kinetic studies on LRRKtide and its analog LRRKtide(S). Their phosphorylation differs by the rate-limiting steps: product release is rate-limiting for LRRKtide and phosphoryl transfer is rate-limiting for LRRKtide(S). As a result, we observed that the I2020T mutant is more active than wild type (WT) LRRK2 for LRRKtide(S) phosphorylation, whereas it is less active than WT for LRRKtide phosphorylation. Our pre-steady-state kinetic data suggest that (i) the I2020T mutant accelerates the rates of phosphoryl transfer of both reactions by 3-7-fold; (ii) this increase is masked by a rate-limiting product release step for LRRKtide phosphorylation; and (iii) the observed lower activity of the mutant for LRRKtide phosphorylation is a consequence of its instability: the concentration of the active form of the mutant is 3-fold lower than WT. The I2020T mutant has a dramatically low KATP and therefore leads to resistance to ATP competitive inhibitors. Two well known DFG-out or type II inhibitors are also weaker toward the mutant because they inhibit the mutant in an unexpected ATP competitive mechanism. The I2020 residue lies next to the DYG motif of the activation loop of the LRRK2 kinase domain. Our modeling and metadynamic simulations suggest that the I2020T mutant stabilizes the DYG-in active conformation and creates an unusual allosteric pocket that can bind type II inhibitors but in an ATP competitive fashion.
Keywords: Enzyme Inhibitors; Enzyme Kinetics; LRRK2; Molecular modeling; Parkinson Disease.
Figures





References
-
- Moore D. J., West A. B., Dawson V. L., Dawson T. M. (2005) Molecular pathophysiology of Parkinson's disease. Annu. Rev. Neurosci. 28, 57–87 - PubMed
-
- Paisán-Ruíz C., Jain S., Evans E. W., Gilks W. P., Simón J., van der Brug M., López de Munain A., Aparicio S., Gil A. M., Khan N., Johnson J., Martinez J. R., Nicholl D., Carrera I. M., Pena A. S., de Silva R., Lees A., Martí-Massó J. F., Pérez-Tur J., Wood N. W., Singleton A. B. (2004) Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron 44, 595–600 - PubMed
-
- Zimprich A., Biskup S., Leitner P., Lichtner P., Farrer M., Lincoln S., Kachergus J., Hulihan M., Uitti R. J., Calne D. B., Stoessl A. J., Pfeiffer R. F., Patenge N., Carbajal I. C., Vieregge P., Asmus F., Müller-Myhsok B., Dickson D. W., Meitinger T., Strom T. M., Wszolek Z. K., Gasser T. (2004) Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44, 601–607 - PubMed
-
- Berg D., Schweitzer K. J., Leitner P., Zimprich A., Lichtner P., Belcredi P., Brüssel T., Schulte C., Maass S., Nägele T., Wszolek Z. K., Gasser T. (2005) Type and frequency of mutations in the LRRK2 gene in familial and sporadic Parkinson's disease. Brain 128, 3000–3011 - PubMed
-
- Khan N. L., Jain S., Lynch J. M., Pavese N., Abou-Sleiman P., Holton J. L., Healy D. G., Gilks W. P., Sweeney M. G., Ganguly M., Gibbons V., Gandhi S., Vaughan J., Eunson L. H., Katzenschlager R., Gayton J., Lennox G., Revesz T., Nicholl D., Bhatia K. P., Quinn N., Brooks D., Lees A. J., Davis M. B., Piccini P., Singleton A. B., Wood N. W. (2005) Mutations in the gene LRRK2 encoding dardarin (PARK8) cause familial Parkinson's disease: clinical, pathological, olfactory and functional imaging and genetic data. Brain 128, 2786–2796 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical
Molecular Biology Databases
Research Materials

