Discovery of biomarkers for glycaemic deterioration before and after the onset of type 2 diabetes: rationale and design of the epidemiological studies within the IMI DIRECT Consortium
- PMID: 24695864
- PMCID: PMC4018481
- DOI: 10.1007/s00125-014-3216-x
Discovery of biomarkers for glycaemic deterioration before and after the onset of type 2 diabetes: rationale and design of the epidemiological studies within the IMI DIRECT Consortium
Abstract
Aims/hypothesis: The DIRECT (Diabetes Research on Patient Stratification) Study is part of a European Union Framework 7 Innovative Medicines Initiative project, a joint undertaking between four industry and 21 academic partners throughout Europe. The Consortium aims to discover and validate biomarkers that: (1) predict the rate of glycaemic deterioration before and after type 2 diabetes onset; (2) predict the response to diabetes therapies; and (3) help stratify type 2 diabetes into clearly definable disease subclasses that can be treated more effectively than without stratification. This paper describes two new prospective cohort studies conducted as part of DIRECT.
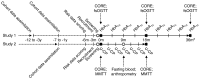
Methods: Prediabetic participants (target sample size 2,200-2,700) and patients with newly diagnosed type 2 diabetes (target sample size ~1,000) are undergoing detailed metabolic phenotyping at baseline and 18 months and 36 months later. Abdominal, pancreatic and liver fat is assessed using MRI. Insulin secretion and action are assessed using frequently sampled OGTTs in non-diabetic participants, and frequently sampled mixed-meal tolerance tests in patients with type 2 diabetes. Biosamples include venous blood, faeces, urine and nail clippings, which, among other biochemical analyses, will be characterised at genetic, transcriptomic, metabolomic, proteomic and metagenomic levels. Lifestyle is assessed using high-resolution triaxial accelerometry, 24 h diet record, and food habit questionnaires.
Conclusions/interpretation: DIRECT will yield an unprecedented array of biomaterials and data. This resource, available through managed access to scientists within and outside the Consortium, will facilitate the development of new treatments and therapeutic strategies for the prevention and management of type 2 diabetes.
Figures

References
-
- Ajanki T (2013) ANDIS-Alla Nya Diabetiker i Skåne. http://diabetesportalen.se/foerdjupning/viktiga-vetenskapliga-undersoekn.... Accessed 15 Oct 2013
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

