Magnesium supplementation in pregnancy
- PMID: 24696187
- PMCID: PMC6507506
- DOI: 10.1002/14651858.CD000937.pub2
Magnesium supplementation in pregnancy
Abstract
Background: Magnesium is an essential mineral required for regulation of body temperature, nucleic acid and protein synthesis and in maintaining nerve and muscle cell electrical potentials. Many women, especially those from disadvantaged backgrounds, have low intakes of magnesium. Magnesium supplementation during pregnancy may be able to reduce fetal growth restriction and pre-eclampsia, and increase birthweight.
Objectives: To assess the effects of magnesium supplementation during pregnancy on maternal, neonatal/infant and paediatric outcomes.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 March 2013).
Selection criteria: Randomised and quasi-randomised trials assessing the effects of dietary magnesium supplementation during pregnancy were included. The primary outcomes were perinatal mortality (including stillbirth and neonatal death prior to hospital discharge), small-for-gestational age, maternal mortality and pre-eclampsia.
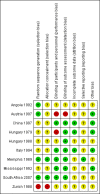
Data collection and analysis: Two review authors independently assessed study eligibility, extracted data and assessed the risk of bias of included studies.
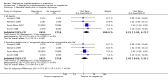
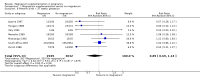
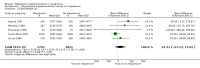
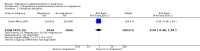
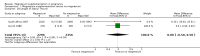
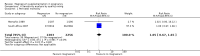
Main results: Ten trials involving 9090 women and their babies were included; one trial had a cluster design (with randomisation by study centre). All 10 trials randomly allocated women to either an oral magnesium supplement or a control group; in eight trials a placebo was used, and in two trials no treatment was given to the control group. In the 10 included trials, the compositions of the magnesium supplements, gestational ages at commencement, and doses administered varied, including: magnesium oxide, 1000 mg daily from ≤ four months post-conception (one trial); magnesium citrate, 365 mg daily from ≤ 18 weeks until hospitalisation after 38 weeks (one trial), and 340 mg daily from nine to 27 weeks' gestation (one trial); magnesium gluconate, 2 to 3 g from 28 weeks' gestation until birth (one trial), and 4 g daily from 23 weeks' gestation (one trial); magnesium aspartate, 15 mmol daily (three trials, commencing from either six to 21 weeks' gestation until birth, ≤ 16 weeks' gestation until birth, or < 12 weeks until birth), or 365 mg daily from 13 to 24 weeks until birth (one trial); and magnesium stearate, 128 mg elemental magnesium from 10 to 35 weeks until birth (one trial).In the analysis of all trials, oral magnesium supplementation compared to no magnesium was associated with no significant difference in perinatal mortality (stillbirth and neonatal death prior to discharge) (risk ratio (RR) 1.10; 95% confidence interval (CI) 0.72 to 1.67; five trials, 5903 infants), small-for-gestational age (RR 0.76; 95% CI 0.54 to 1.07; three trials, 1291 infants), or pre-eclampsia (RR 0.87; 95% CI 0.58 to 1.32; three trials, 1042 women). None of the included trials reported on maternal mortality.Considering secondary outcomes, while no increased risk of stillbirth was observed, a possible increased risk of neonatal death prior to hospital discharge was shown for infants born to mothers who had received magnesium (RR 2.21; 95% CI 1.02 to 4.75; four trials, 5373 infants). One trial contributed over 70% of the participants to the analysis for this outcome; the trial authors suggested that the large number of severe congenital anomalies in the supplemented group (unlikely attributable to magnesium) and the deaths of two sets of twins (with birthweights < 750 g) in the supplemented group likely accounted for the increased risk of death observed, and thus this result should be interpreted with caution. Furthermore, when the deaths due to severe congenital abnormalities in this trial were excluded from the meta-analysis, no increased risk of neonatal death was seen for the magnesium supplemented group. Magnesium supplementation was associated with significantly fewer babies with an Apgar score less than seven at five minutes (RR 0.34; 95% CI 0.15 to 0.80; four trials, 1083 infants), with meconium-stained liquor (RR 0.79; 95% CI 0.63 to 0.99; one trial, 4082 infants), late fetal heart decelerations (RR 0.68; 95% CI 0.53 to 0.88; one trial, 4082 infants), and mild hypoxic-ischaemic encephalopathy (RR 0.38; 95% CI 0.15 to 0.98; one trial, 4082 infants). Women receiving magnesium were significantly less likely to require hospitalisation during pregnancy (RR 0.65, 95% CI 0.48 to 0.86; three trials, 1158 women).Of the 10 trials included in the review, only two were judged to be of high quality overall. When an analysis was restricted to these two trials none of the review's primary outcomes (perinatal mortality, small-for-gestational age, pre-eclampsia) were significantly different between the magnesium supplemented and control groups.
Authors' conclusions: There is not enough high-quality evidence to show that dietary magnesium supplementation during pregnancy is beneficial.
Conflict of interest statement
Maria Makrides ‐ has received advisory board payments from Nestle Nutrition Insitute, Fonterra,and Nutricia/Danone. These advisory boards were related to clinical nutrition in paediatric settings (and therefore not related to the topic under review). The Nestle Nutrition Institute advisory board was only focused on education and training aspects and there were no discussions relating to products. All the honoraria associated with these boards were paid to Maria Makrides' institution and used to fund continuing education and travel for students, early and mid‐career researchers.
Danielle Crosby ‐ none known
Emily Shepherd ‐ none known
Caroline Crowther ‐ none known
Figures































Update of
-
Magnesium supplementation in pregnancy.Cochrane Database Syst Rev. 2001;(4):CD000937. doi: 10.1002/14651858.CD000937. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2014 Apr 03;(4):CD000937. doi: 10.1002/14651858.CD000937.pub2. PMID: 11687087 Updated.
References
References to studies included in this review
Angola 1992 {published data only}
-
- D'Almeida A, Carter JP, Antol A, Prost C. Effects of a combination of evening primrose oil (gamma linolenic acid) and fish oil (eicosapentaenoic + docosahexaenoic acid) versus magnesium, and placebo in preventing pre‐eclampsia. Women and Health 1992;19:117‐31. - PubMed
Austria 1997 {published data only}
-
- Arikan G, Gucer F, Scholl W, Weiss PAM. Preterm labour during oral magnesium supplementation in uncomplicated pregnancies [Fruhgeburtlichkeit unter oraler magnesium substitution bei unkomplizierten schwangerschaften: eine randomisiert kontrolliert klinische studie]. Geburtshilfe und Frauenheilkunde 1997;57:491‐5.
-
- Arikan G, Panzitt T, Gaucer F, Boritsch J, Trojovski A, Haeusler MCH. Oral magnesium supplementation and the prevention of preterm labor. American Journal of Obstetrics and Gynaecology 1997;176:(1Pt2):S45.
China 1997 {published data only}
-
- Li S, Tian H. Oral low‐dose magnesium gluconate preventing pregnancy induced hypertension (translation). Chunh‐Hua‐Fu‐Chan‐Ko‐Tsa‐Chih [Chinese Journal of Obstetrics and Gynaecology] 1997;32:613‐5. - PubMed
Hungary 1979 {published data only}
-
- Balazs M, Kuti V, Morvay F, Varenka Zs, Szekely A, Szucs M. New data on the relationship between magnesium intake and the quality of pregnancy [Données nouvelles sur les relations entre l'apport alimentaire en magnésium et la qualité de la grossesse]. Revue Française d'Endocrinologie Clinique, Nutrition, et Métabolisme 1980;21(6):525‐7.
-
- Balazs M, Morvai F, Szekely Z, Miklos Szucs, Varenka Z, Kuti V. Miscarriage and magnesium intake [Avortment spontané et apport en magnésium de la ration alimentaire]. Revue Française d'Endocrinologie Clinique, Nutrition, et Métabolisme 1979;20(6):525‐9.
Hungary 1988 {published data only}
-
- Kovacs L, Molnar BG, Huhn E, Bodis L. Magnesium substitution in pregnancy. A prospective, randomized double‐blind study [Magnesium substitution in der Schwangerschaft: eine prospektive, randomisierte doppelblindstudie]. Geburtshilfe und Frauenheilkunde 1988;48:595‐600. - PubMed
-
- Kovacs L, Molnar GB, Huhn E, Bodis L. Magnesium substitution during pregnancy. A prospective randomised, double blind, clinical trial. Magyar Noorvosok Lapja 1988;51:9‐14.
Italy 1994 {published data only}
-
- Zarcone R, Cardone G, Bellini P. Role of magnesium in pregnancy. Panminerva Medica 1994;36:168‐70. - PubMed
Memphis 1989 {published data only}
-
- Sibai BM, Villar MA, Bary E. Magnesium supplementation during pregnancy: a double‐blind randomized controlled clinical trial. American Journal of Obstetrics and Gynaecology 1989;161:115‐9. - PubMed
Mississippi 1992 {published data only}
-
- Martin RW, McColgin SW, Perry KG, McCaul JF, Hess LW, Martin JN, et al. Oral magnesium and the prevention of preterm labor in a high‐risk group of patients. Proceedings of 10th Annual Meeting of Society of Perinatal Obstetricians, Houston, Texas, USA. 1990:181.
-
- Martin RW, Perry KG, Hess LW, Martin JN, Morrison JC. Oral magnesium and the prevention of preterm labor in a high‐risk group of patients. American Journal of Obstetrics and Gynaecology 1992;166:144‐7. - PubMed
South Africa 2007 {published data only}
-
- Fawcus S, Harrison V, Jordaan E. The prevention of neonatal encephalopathy: could magnesium supplementation during pregnancy play a role? [abstract]. 31st British International Congress of Obstetrics and Gynaecology. London, UK, 2007 July 4‐6:102.
-
- Harrison V, Fawcus S, Jordaan E. Magnesium supplementation and perinatal hypoxia: outcome of a parallel group randomised trial in pregnancy. British Journal of Obstetrics and Gynaecology 2007;114:994‐1002. - PubMed
Zurich 1988 {published data only}
-
- Jaspers V, Spatling L, Fallenstein F, Quakernack K. Magnesium, calcium, haemoglobin, haematocrit, oestriol and HPL following magnesium substitution [supplementation] during pregnancy [Magnesium, kalzium, hamoglobin, hamatokrit, ostriol und HPL unter magnesium substitution in der schwangerschaft]. Geburtshilfe und Frauenheilkunde 1990;50:628‐33. - PubMed
-
- Spatling L. Magnesium supplementation in pregnancy in pregnancy: a double‐blind study. Proceedings of International Symposium on Advances in the Prevention of Low Birthweight, Cape Cod, Massachusetts, USA. 1988:197‐201.
-
- Spatling L, Spatling G. Magnesium supplementation in pregnancy. A double‐blind study. British Journal of Obstetrics and Gynaecology 1988;95:120‐5. - PubMed
-
- Vetter K, Spaetling L. Magnesium substitution in pregnancy a double‐blind study [Magnesium substitution in der schwangerschaft eine doppelblindstudie]. Archives of Gynaecology 1986;239:176‐7.
References to studies excluded from this review
Denmark 1990 {published data only}
-
- Rudnicki M, Junge J, Frolich A, Ornvold K, Fischer‐Rasmussen W. Magnesium supplement in pregnancy‐induced hypertension A clinicopathological study. Acta Pathologica, Microbiologica, et Immunologica Scandinavica 1990;98:1123‐7. - PubMed
Denmark 1991 {published data only}
-
- Rudnicki M, Frølich A, Fischer‐Rasmussen W. Magnesium supplement in pregnancy‐induced hypertension: effects on maternal and neonatal magnesium and calcium homeostasis. Mineral and Electrolyte Metabolism 1991;17(6):399‐403. - PubMed
Detroit 1999 {published data only}
-
- Hallak M, Martinez‐Poyer J, Brish L, Poole‐Bryant K, Mammen EF, Sorokin Y. Magnesium sulfate impairs platelet function in normal pregnant women. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S142.
-
- Hallak M, Martinez‐Poyer J, Kruger M, Hassan S, Blackwell S, Sorokin Y. The effect of magnesium sulfate on fetal heart rate parameters: A randomized, placebo‐controlled trial. American Journal of Obstetrics and Gynecology 1999;181(5):1122‐7. - PubMed
-
- Hallak M, Martinez‐Poyer J, Kruger ML, Hassan S, Blackwell SC, Sorokin Y. Magnesium sulfate effect on fetal heart rate (FHR) parameters: A randomized, placebo controlled trial. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S155. - PubMed
-
- Hallak M, Martinez‐Poyer J, Kruger ML, King M, Russell E, Sorokin Y. Magnesium sulfate transiently increases fetal breathing movements but not body movements. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S155.
-
- Martinez‐Poyer J, Hallak M, King M, Kruger M, Sorokin Y. Magnesium sulfate increases impedance to flow in the fetal systemic circulation but has no effect on the cerebral circulation. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S114.
India 2012 {published data only}
-
- Dasgupta S, Ghosh D, Seal SL, Kamilya G, Karmakar M, Saha D. Randomized controlled study comparing effect of magnesium sulfate with placebo on fetal umbilical artery and middle cerebral artery blood flow in mild preeclampsia at ≥ 34 weeks gestational age. Journal of Obstetrics and Gynaecology Research 2012;38(5):763‐71. - PubMed
ISRCTN03989660 {published data only}
-
- ISRCTN03989660. A randomized, double‐blinded, placebo‐controlled trial of oral magnesium for relief in pregnancy‐induced leg cramps. http://www.controlled‐trials.com/ISRCTN03989660/ (accessed 7 February 2013).
NCT01709968 {published data only}
-
- NCT01709968. Magnesium oxide monohydrate for nocturnal leg cramps (mgnlc); a prospective, randomized, double blind, placebo controlled clinical trial. http://clinicaltrials.gov/ct2/show/NCT01709968 (accessed 7 February 2013).
Norway 2008 {published data only}
-
- Nygaard IH, Valbø A, Pethick SV, Bøhmer T. Does oral magnesium substitution relieve pregnancy‐induced leg cramps?. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2008;141(1):23‐6. - PubMed
Sweden 1987 {published data only}
-
- Hammar M, Berg G, Solheim F, Larsson L. Calcium and magnesium status in pregnant women. A comparison between treatment with calcium and vitamin C in pregnant women with leg cramps. International Journal of Vitamin and Nutrition Research 1987;57:179‐83. - PubMed
Sweden 1995 {published data only}
-
- Dahle LO, Berg G, Hammar M, Hurtig M, Larsson L. The effect of oral magnesium substitution on pregnancy‐induced leg cramps. American Journal of Obstetrics and Gynecology 1995;173(1):175‐80. - PubMed
References to ongoing studies
ISRCTN98365455 {published data only}
-
- ISRCTN98365455. Magnesium intervention during pregnancy. http://www.controlled‐trials.com/isrctn/pf/98365455 (accessed 25 August 2011).
NCT01510665 {published data only}
-
- NCT01510665. Magnesium supplementation in the second trimester of pregnancy to overweight and obese individuals. http://clinicaltrials.gov/ct2/show/NCT01510665 (accessed 7 February 2013).
Additional references
Arikan 1999
-
- Arikan GM, Panzitt T, Gücer F, Scholz HS, Reinisch S, Haas J, et al. Course of maternal serum magnesium levels in low‐risk gestations and in preterm labor and delivery. Fetal Diagnosis and Therapy 1999;14(6):332‐6. - PubMed
Conradt 1984
-
- Conradt A, Weidinger H, Algayer. On the role of magnesium in fetal hypotrophy, pregnancy induced hypertension and pre‐eclampsia. Magnesium Bulletin 1984;6:68‐76.
Doyle 1989
-
- Doyle W, Crawford MA, Wynn AH, Wynn SW. Maternal magnesium intake and pregnancy outcome. Magnesium Research 1989;2:205‐10. - PubMed
Duley 2010
Garrison 2012
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Inst Med 1990
-
- Subcommittee on Nutritional Status and Weight Gain During Pregnancy, Subcommittee on Dietary Intake and Nutrient Supplements During Pregnancy, Committee on Nutritional Status During Pregnancy and Lactation, Food, Nutrition Board. Institute of Medicine, National Academy of Sciences. Nutrition During Pregnancy. Washington DC: National Academy Press, 1990.
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
US FDA 2009
-
- US Department of Health and Human Services: US FDA. Overview of dietary supplements: What is a dietary supplement?. http://www.fda.gov/Food/DietarySupplements/ConsumerInformation/ucm110417... 2009 [accessed 25 Aug 2011].
References to other published versions of this review
Keirse 1995
-
- Keirse MJNC. Routine magnesium supplementation in pregnancy. Pregnancy and Childbirth Module; The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Oxford, 1995 [revised 16 June 1993], issue 2.
Makrides 1998
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

