Disease manifestations and pathogenic mechanisms of Group A Streptococcus
- PMID: 24696436
- PMCID: PMC3993104
- DOI: 10.1128/CMR.00101-13
Disease manifestations and pathogenic mechanisms of Group A Streptococcus
Abstract
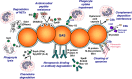
Streptococcus pyogenes, also known as group A Streptococcus (GAS), causes mild human infections such as pharyngitis and impetigo and serious infections such as necrotizing fasciitis and streptococcal toxic shock syndrome. Furthermore, repeated GAS infections may trigger autoimmune diseases, including acute poststreptococcal glomerulonephritis, acute rheumatic fever, and rheumatic heart disease. Combined, these diseases account for over half a million deaths per year globally. Genomic and molecular analyses have now characterized a large number of GAS virulence determinants, many of which exhibit overlap and redundancy in the processes of adhesion and colonization, innate immune resistance, and the capacity to facilitate tissue barrier degradation and spread within the human host. This improved understanding of the contribution of individual virulence determinants to the disease process has led to the formulation of models of GAS disease progression, which may lead to better treatment and intervention strategies. While GAS remains sensitive to all penicillins and cephalosporins, rising resistance to other antibiotics used in disease treatment is an increasing worldwide concern. Several GAS vaccine formulations that elicit protective immunity in animal models have shown promise in nonhuman primate and early-stage human trials. The development of a safe and efficacious commercial human vaccine for the prophylaxis of GAS disease remains a high priority.
Figures










References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

